Synthesis of Esters
Synthesis of Esters
Esters can be synthesized by different methods. Some of the most common methods for the synthesis of esters are discussed below.
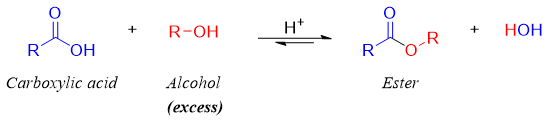
Synthesis of esters from carboxylic acids (Fischer Esterification).
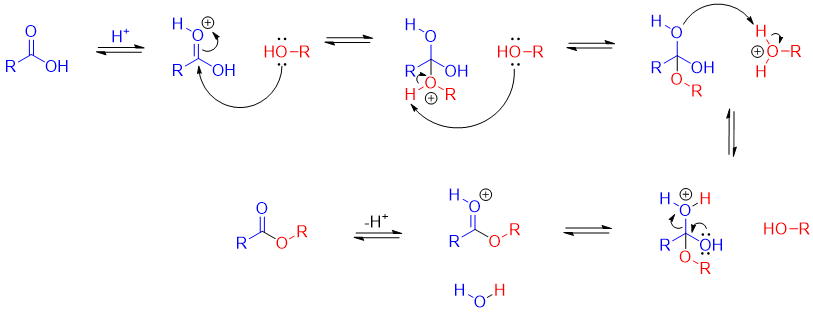
This method of synthesizing an ester from carboxylic acid by treatment of alcohols was first discovered by Emil Fischer. The reaction was carried out in the presence of acid catalyst. The tetrahedral intermediate formed in this reaction has two possible leaving groups (i.e. -OH and -OR) and both having almost the same basicities therefore, the alcohol is reacted in excess.
Mechanism:
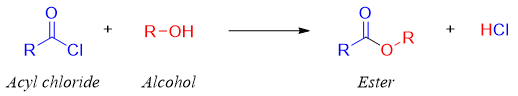
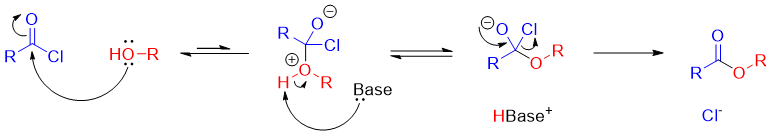
Synthesis of esters from acyl chlorides:
Esters are usually synthesized by treating alcohols with acyl chlorides. In this rection the nucleophile (alcohol) is the stronger base than the departing leaving group (chloride).
Mechanism:
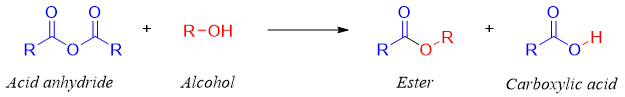
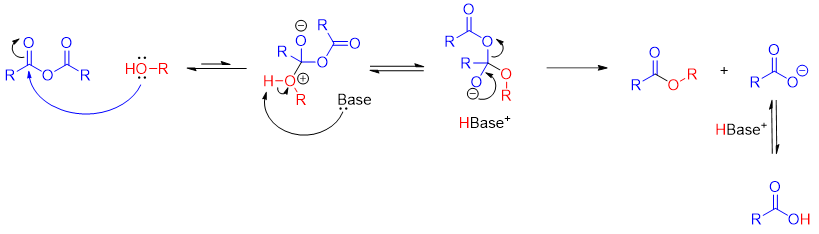
Synthesis of esters from acid anhydride:
Esters can be synthesized by reacting alcohols with acid anhydrides. The byproduct of this reaction is carboxylate ion which is a stronger base, and it abstracts proton to form corresponding carboxylic acid.
Mechanism:
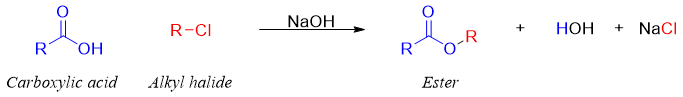
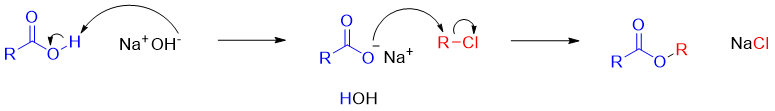
Synthesis of esters from alkyl halides:
Esters are also synthesized from the sodium salts of carboxylic acids and alkyl chlorides. This reaction is limited to primary alkyl halides.
Mechanism:
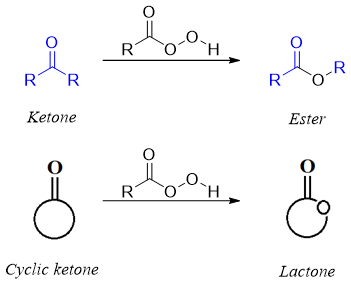
Synthesis of esters from ketones (Baeyer–Villiger oxidation):
This reaction involves the synthesis of esters or lactones from ketones and cyclic ketones respectively in the presence of peroxides or peroxyacids.
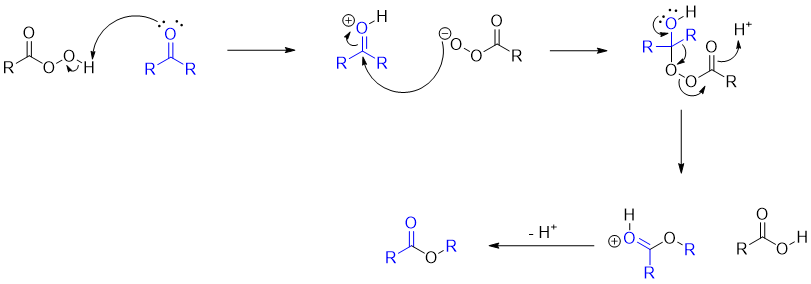
Mechanism:
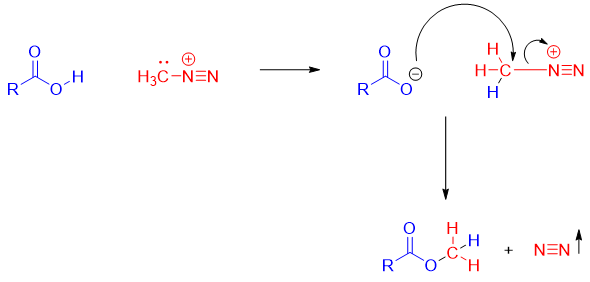
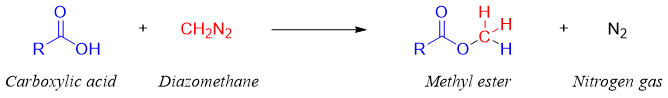
Synthesis of methyl esters from diazomethane:
Methyl esters can be synthesized from diazomethane when treated with carboxylic acids.
Mechanism: