Nomenclature of Esters
Nomenclature of Esters
Esters are the derivatives of carboxylic acids in which the hydrogen atom of hydroxyl group (-OH) is replaced by an alkyl group R.
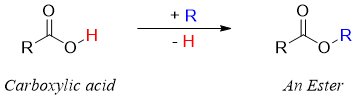
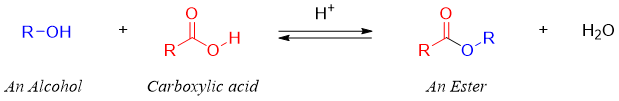
Most commonly esters are synthesized by reacting alcohols with carboxylic acids. The -OH group of carboxylic acid is replaced by the alkoxide group (-OR) of alcohols to produce esters. This reaction is known as Esterification reaction.
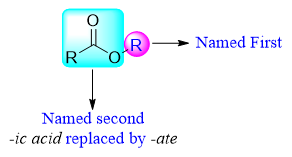
Esters are named in a systematic way. The name of the alkyl is named first followed by the name of carboxylic acid. The name “ic acid” of carboxylic acid is replaced by “ate”.
For example, the following ester is named by first identifying the alkyl group (-R) and then identifying the carboxylic group.
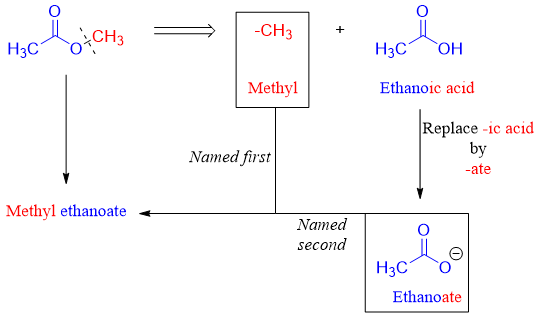
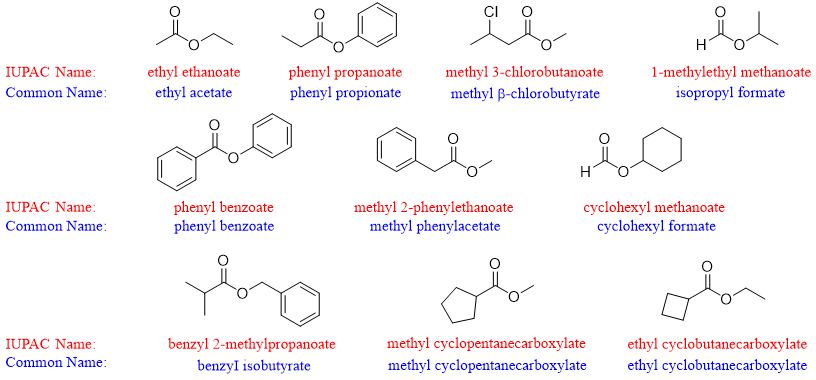
Following are some examples of esters. Both IUPAC and common names are assigned.
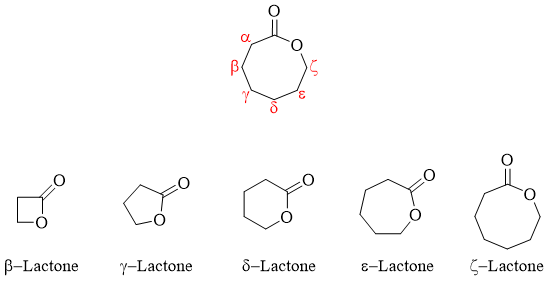
Esters in cyclic form are called as Lactones. Lactones are formed when the hydroxyl group reacts with the carboxylic group of the same compound.

In IUPAC naming lactones are named as “2-oxacycloalkanones”. The numbering is started from carbonyl carbon.
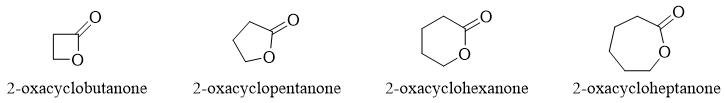
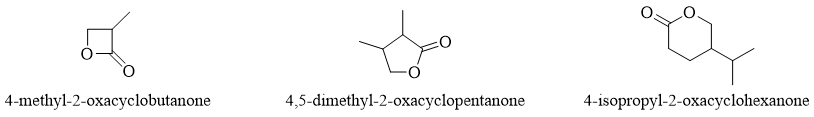
Substituted lactones are named starting numbering from carbonyl carbon and giving second number to oxa oxygen and so on.
Lactones are also systematically named by placing word lactone at the end of the name of the hydroxy parent acid.
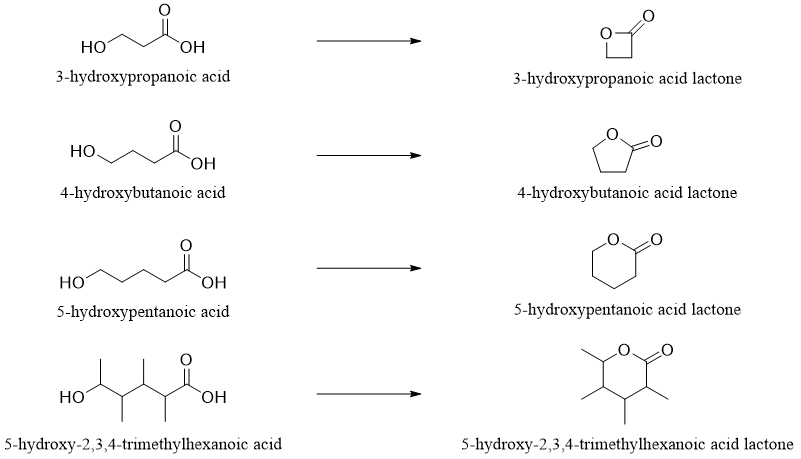
Sometimes the length of carbon chain in lactones is designated by the Greek letter. The Greek letter specifies the carbon atom to which the oxygen (oxa) is attached.