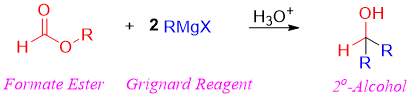Esters Addition of Grignard reagents
Addition of Grignard Reagent
Esters react with two equivalents of Grignard reagent to produce tertiary alcohols.
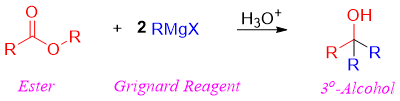
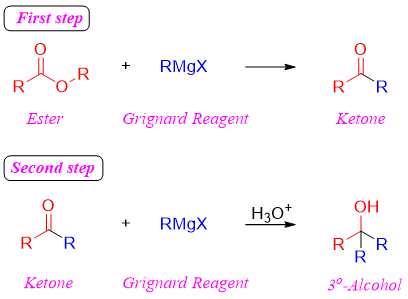
Two successive reactions take place when Grignard reagent is reacted with ester. The first reaction is the nucleophilic acyl substitution reaction in which the alkoxy group of esters is replaced by an alkyl group resulting in the formation of ketones. In the second step, nucleophilic addition reaction take place forming tertiary alcohols.
The first involves the production of a unstable tetrahedral intermediate. This unstable intermediate expels the alkoxide group to produce a ketone. The ketone produced further reacts with the second equivalent of Grignard reagent. Ketones are more reactive than esters hence, it is not possible to stop the reaction at ketone stage. The reaction of ketone with Grignard reagent results in the formation of magnesium salt of tertiary alkoxide. This salt when treated with aqueous acid produces tertiary alcohol. The tertiary alcohols contain three alkyl groups, one derived from the ester and other two derived from the Grignard reagent. Therefore, tertiary alcohols synthesized from the reaction of an ester and Grignard reagents will always contain two similar alkyl groups.
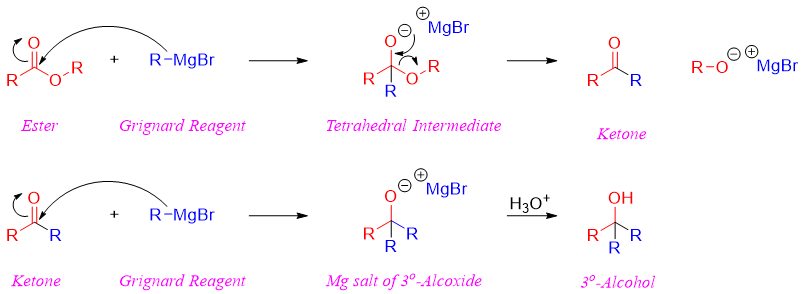
Incase if ester is reacted with only one mole of Grignard reagent it will produce a mixture of products. This mixture will include ketone, tertiary alcohol, and unreacted ester.
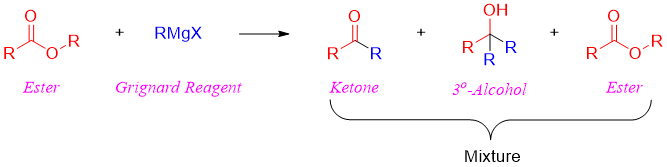
Meanwhile, formate esters when reacted with Grignard reagent forms secondary alcohols with hydrogen derived from ester and two alkyl groups derived from Grignard reagent.