Hydrolysis - Conversion Esters to Carboxylic Acid
Hydrolysis of an Esters
Esters react with water to produce a carboxylic acid and an alcohol. This reaction is called as hydrolysis of an ester. Reaction of ester with water is a slow reaction because the water molecule is a weak nucleophile and the leaving group i.e., RO- is a poor leaving group as it is strongly basic in nature. To increase the rate of hydrolysis reaction either acid or base is added as catalyst.
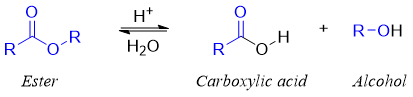
Acid-catalyzed hydrolysis of an ester:
In acidic medium the reactants, intermediates and the final products are either neutral or positively charged. No negatively charged species are found in acidic solutions. The general reaction for the hydrolysis of an ester in acidic solution is given below.
Mechanism:
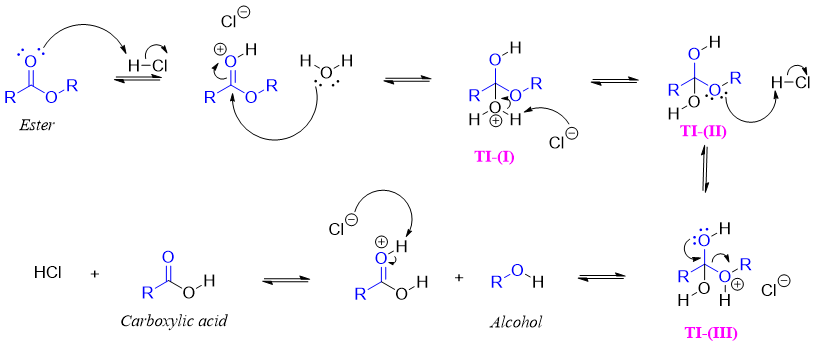
In above mechanism three tetrahedral intermediates (TI) are shown. The TI-I can collapse and can reform the ester as the water is a weak base (good leaving group). Since the conjugate base of acid can abstract the proton from (H2O)+ and generate TI-II. TI-II does not collapse as both the -OH and -OR are weak leaving groups due to their high basicities. The tetrahedral intermediate III on collapse produces carboxylic acid because ROH is a good leaving group and a weaker base.
Hence, acid catalyzes the hydrolysis reaction by protonating the carbonyl oxygen making the carbonyl carbon more electrophilic and protonating the -OR group (weak leaving group) to ROH a good leaving group.
Since the hydrolysis of ester is a reversible reaction, both ester are carboxylic acid are present in the reaction mixture at equilibrium. To force the reaction to move in forward direction (more carboxylic acid) excess water can be used (Le Châtelier’s principle). Or if the alcohol produced during the reaction is removed by boiling it (alcohols have lower boiling points than acids) will also shift the reaction in forward direction.
The hydrolysis of esters having tertiary alkyl group produces same products i.e., carboxylic acid and alcohol.
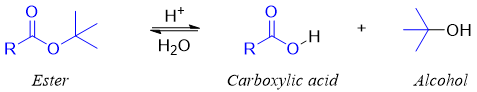
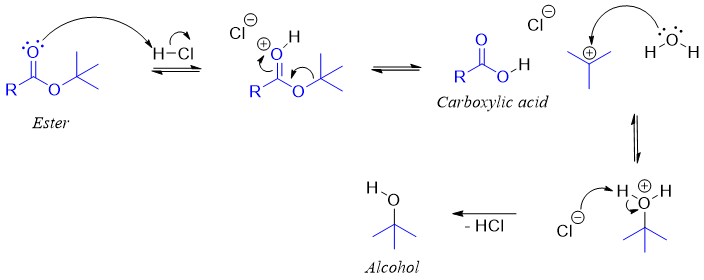
Esters with tertiary alkyl group does not show same mechanism as that shown by esters of primary alkyl halides.
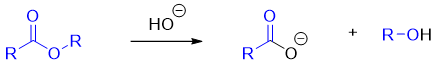
Another method to catalyze hydrolysis of an ester is to add hydroxide ion in the reaction mixture. Hydroxide ion increases the rate of formation of tetrahedral intermediate and the rate of collapse of tetrahedral intermediate. The general hydroxide mediated reaction of ester hydrolysis is shown below.
Mechanism:
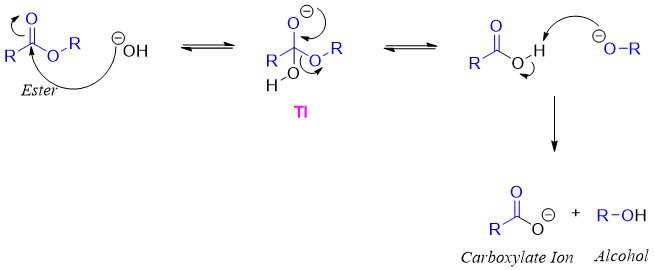
Base increases the rate of hydrolysis of ester as the hydroxide ion is a good nucleophile as compared to water nucleophile. As shown in above mechanism the tetrahedral intermediate (TI) is negatively charged. The rate of elimination of RO- group by negatively charged oxygen is faster than the elimination aided by neutral oxygen atom. Furthermore, the final product is carboxylate ion which is negatively charged specie therefore, it can not be attacked by another nucleophile to reverse the reaction.
The hydroxide ion only promotes the hydrolysis of esters. It can not be used as a catalyst to promote reactions between carboxylic acids and alcohols. This is because the hydroxide ion is used as good nucleophile in the first step of hydrolysis of esters.
