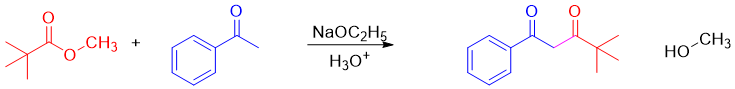Carbon Nucleophiles
Carbon Nucleophiles
Carbon nucleophiles are carbon containing molecules that seeks a positive center (nucleus loving) of an atom in a chemical reaction. These nucleophiles contain a lone pair of electrons which are available for the bond formation. Hence, many nucleophiles that react with esters have their nucleophilic center on carbon atom. Following are some examples of carbon centered nucleophiles.
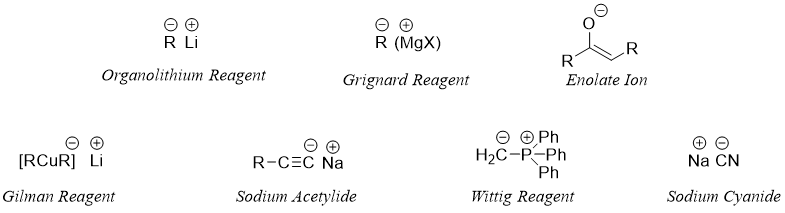
Organolithium reagents have lithium-carbon bond. This bond is highly polar due to high electronegativity difference. Carbon being more electronegative attains partial negative charge hence, making it a good nucleophile.
Esters react with two equivalents of RLi to give tertiary alcohols.
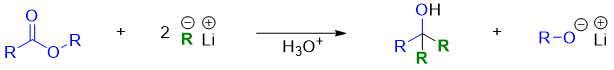
Mechanism:
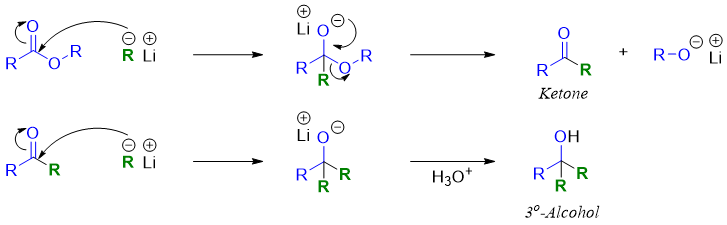
As shown in mechanism this reaction proceeds via a ketone intermediate. The reaction does not stop at ketone stage because ketone is more reactive than the ester. The tertiary alcohol formed in this reaction has two identical alkyl groups. Some undesired products can also form during this reaction. These unwanted products include alkane which are formed by the reaction of RLi with water or any other hydroxyl group present in the reaction mixture. Secondly, a dimer alkane (R-R) can also form when the two alkyl groups from RLi couples.
Grignard reagents react in the same way as organolithium reagent do. Grignard regent reacts with aldehydes to produce secondary alcohols and with ketones and esters it gives tertiary alcohols.
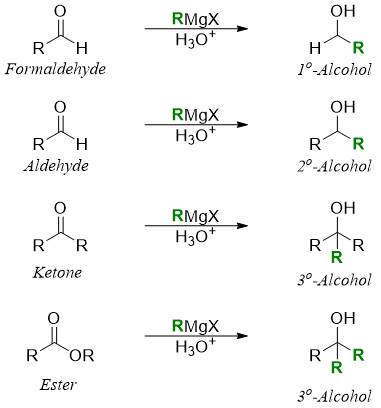
It should be noted that the tertiary alcohols produced from ester always contain to similar alkyl groups.
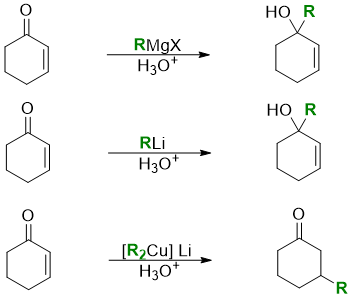
Next carbon nucleophile in our list is Gilman reagent. Gilman reagent contains both lithium and copper metals with alkyl groups. Unlike organolithium reagent and Grignard reagent the carbon nucleophile of Gilman reagent is a soft nucleophile. Therefore, when reacted with alpha beta unsaturated compounds they undergo 1,4 addition rather than the 1,2 addition as that depicted by RLi and RMgX.
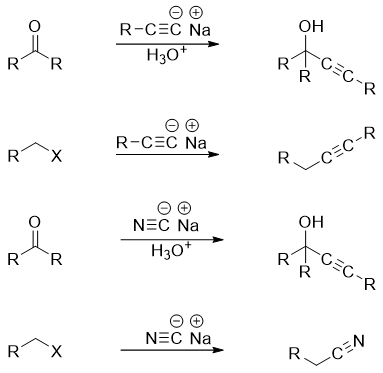
Sodium acetylide is an alkyne carbon nucleophile . This nucleophile reacts with alkyl halides and carbonyl compounds. The nucleophile cyanide also reacts in the same fashion as the acetylide do. Few examples are shown below.
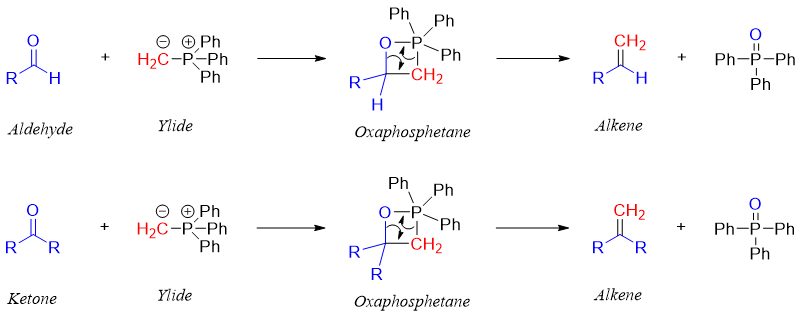
In Wittig reaction a phosphorous ylide is reacted with aldehydes and ketones to synthesize alkenes. This reagent is not useful in reaction with esters. Following are some examples of Wittig reaction.
Another important class of carbon centered nucleophiles are the enolates. Enolates are type of alkenes. Enolates are formed when a carbonyl compound having alpha proton is treated with a base. The base abstracts the alpha hydrogen atom as it is acidic in nature.
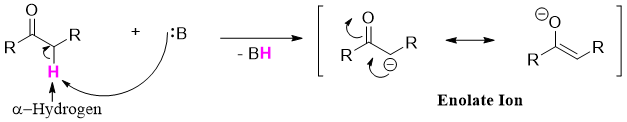
Esters can act as an enolate (nucleophile) or as an electrophile in enolate reactions. These are reactions are called Claisen Condensation reactions. In these reactions the new carbon-carbon bond can form between two identical esters (self-Claisen condensation) or between ester and other carbonyl compound (crossed-Claisen condensation). Following reaction is an example of self-Claisen condensation.
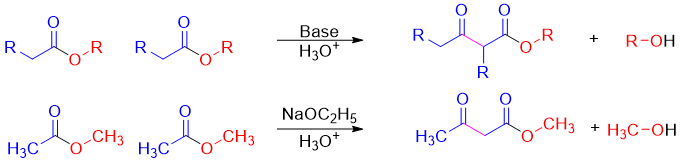
In cross-Claisen condensation two different carbonyl compounds are reacted. The carbonyl compound having alpha hydrogen is converted into an enolate. The second carbonyl compound is often alpha hydrogen less to avoid unwanted byproducts. For example