Synthesis of Carboxylic Acids
Synthesis of Carboxylic Acids
Carboxylic acids can be prepared by many synthetic approaches. Many are quite simple such as the oxidation of alcohols or aldehydes while others are not that simple such as the conversion of alkyl halides into nitriles and their subsequent hydrolysis into carboxylic acids. We will have a look at each one in detail.
Oxidation of Alcohols and Aldehydes
Primary alcohols can be oxidized to carboxylic acids by strong oxidizing agents such as the Jones reagent (CrO3 in H2SO4), potassium dichromate (K2Cr2O7), or sodium hypochlorite (NaOCl).
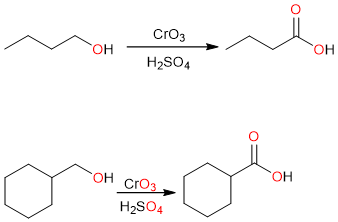
Primary alcohols are not directly oxidized to carboxylic acids, they are first oxidized to aldehydes which are subsequently oxidized to carboxylic acids. The reaction is possible because aldehydes are more sensitive to oxidation than primary alcohols. Secondary and tertiary alcohols do not oxidize to yield carboxylic acids.
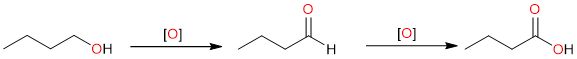
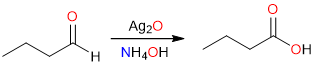
Since aldehydes are easier to oxidize than alcohols, they can be oxidized to carboxylic acids in relatively milder conditions – using mild oxidizing agents.
Oxidative Cleavage of Alkenes and Alkynes
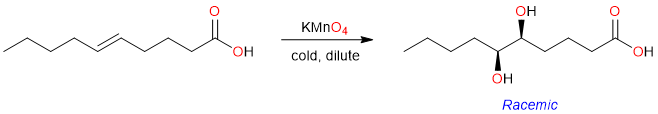
When alkenes are treated with cold dilute potassium permanganate, the reaction yields glycols. The reaction is stereospecific and the two hydroxy groups are always cis to each other. However, the reaction is not enantioselective and the hydroxy groups can either be inserted from the top or the bottom face of the alkene – resulting in the formation of two enantiomers (A racemic product).
When a similar reactant is treated with warm, concentrated KMnO4, the reaction results in the cleavage of C=C bond.

Note that the right side of the alkene bond was disubstituted so that side yielded a ketone while the left side of the double bond (being monosubstituted) yielded a carboxylic acid.
A similar synthesis is the ozonolysis of alkenes followed by an oxidative workup. We know that ozonolysis of alkenes followed by a reductive workup results in the formation of aldehydes and ketones.
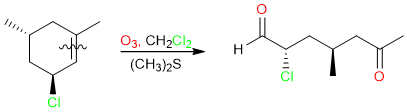
However, the same reaction when performed with oxidative workup yields carboxylic acids. The relationship between alkene substitution and product formation is the same as in KMnO4: The disubstituted side of the alkene yields the ketone and the monosubstituted side of the alkene yields the carboxylic acid.
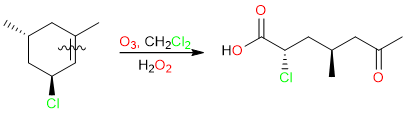
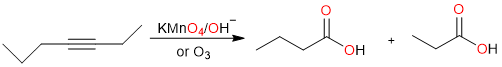
Similarly, alkynes can be oxidatively cleaved into carboxylic acids using either KMnO4 or ozonolysis.
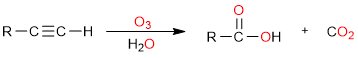
Terminal alkynes however are a bit different. They produce one carboxylic acid and one mole of carbon dioxide.
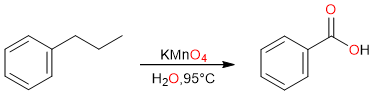
Side-Chain Oxidation of Alkylbenzenes
The side-chain oxidation of an alkylbenzene is another simple way of synthesizing a carboxylic acid.
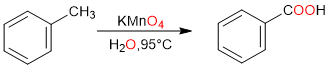
It is important to note that the oxidation takes place at the α-carbon (the carbon atom that is directly attached to the benzene ring). If there is a chain attached to the benzene ring, the α-carbon is oxidized yielding the carboxylic acid while the rest of the alkyl chain is lost during oxidation.
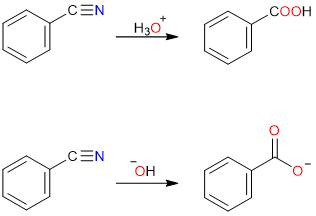
Hydrolysis of Nitriles
Nitriles when hydrolyzed in dilute acid yield carboxylic acids while basic hydrolysis yields carboxylate salts.
It is even possible to convert alkyl halides into carboxylic acids via the nitrile pathway. It is important to note that the carboxylic acid formed has one extra carbon atom (from the CN group).
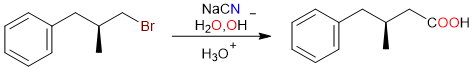
The first step of the reaction is the SN2 substitution of the bromo group by the cyano group.
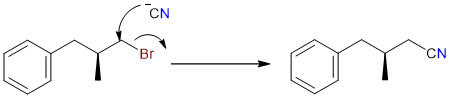
The nitrile thus formed is then hydrolyzed in the basic medium to yield the corresponding carboxylate salt.
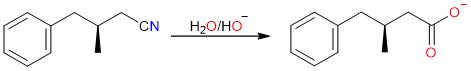
Finally, the acidification step converts the carboxylate salt into a carboxylic acid.
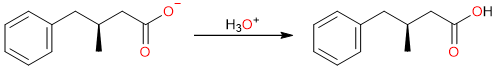
Carboxylation of Grignard Reagents
Another useful method that yields carboxylic acids is the carboxylation of Grignard reagents. Like the last method, the carboxylation of a Grignard reagent results in a carboxylic acid that has one carbon more than the starting material.
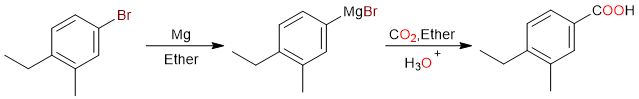
This reaction sequence is pretty simple to understand. The first step is the formation of the Grignard reagent.
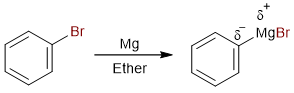
Since magnesium has a lower electronegativity than carbon, the carbon atom is negatively charged. This makes carbon nucleophilic. In the second step, the negatively charged carbon of the Grignard reagent attacks the positively charged carbon of carbon dioxide.
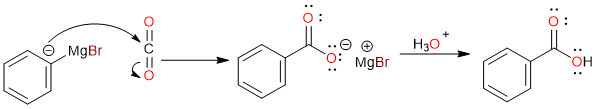
The nucleophilic attack results in the formation of a carboxylate salt, which is then acidified to give the final carboxylic acid.
