Decarboxylation
Introduction to Decarboxylation
A decarboxylation reaction is a reaction in which a carboxylic acid loses its carboxylic acid group as carbon dioxide to yield an alkane (or in some cases an alkyl halide). Most of these reactions are facilitated by heat.
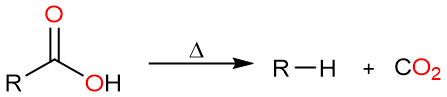
A decarboxylation reaction can be thought of as the reverse of a carboxylation reaction. A carboxylation reaction gives a carboxylic acid that has one carbon more than the starting material. Similarly, a decarboxylation reaction results in the loss of one carbon atom.
Decarboxylation of β-keto Acids
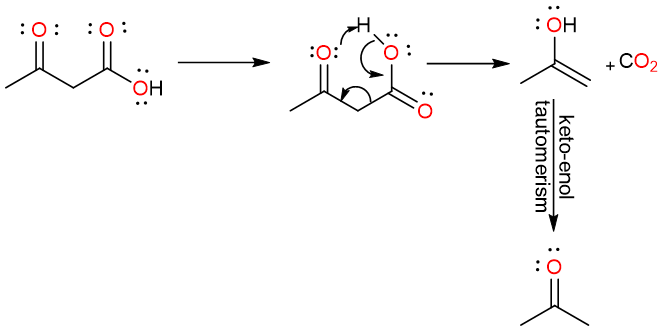
One of the easiest compounds to decarboxylate are the β-keto acids. These are compounds that have another carbonyl group (aldehyde or ketone) one carbon away from the carboxylic acid group.
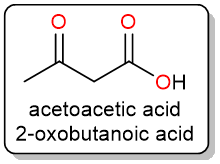
These compounds are easily decarboxylated using gentle heating. The reaction proceeds through a cyclic six-membered concerted mechanism.
Some Famous Decarboxylation Reactions
We will now have a look at a few famous named reactions. All of these reactions proceed through radical mechanisms.
Kolbe Electrolysis
Kolbe electrolysis is the anodic oxidation of a carboxylate salt that yields an alkane. An interesting feature of this reaction is that it gives a dimeric alkane as the product.
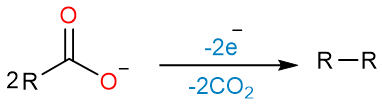
The reaction starts with the loss of one electron by the carboxylate ion on the anode. A carboxylate radical is formed.
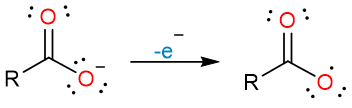
The carboxylate radical loses carbon dioxide to form an alkyl radical.
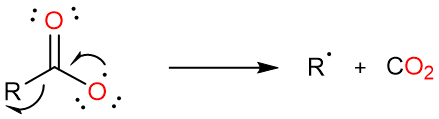
Two alkyl radicals react with each other to give the dimeric alkane.

Barton Decarboxylation
Barton decarboxylation is a two-step process that first converts a carboxylic acid to the Barton ester. Secondly, the Barton ester is then reduced in a radical reduction to an alkane.
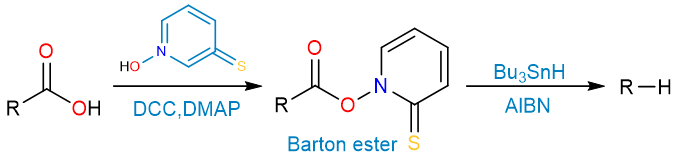
The first step of the reaction is the formation of the Barton ester from the carboxylic acid using DCC and DMAP.
The mechanism of the second step starts with the decomposition of the azoisobutyronitrile (AIBN) molecule to release a nitrogen molecule and two 2-cyanoprop-2-yl radicals.
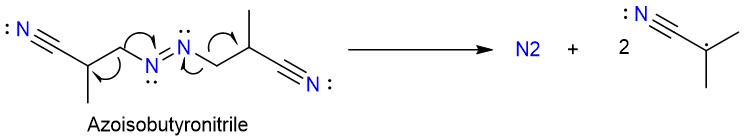
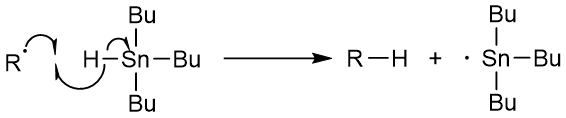
The 2-cyanoprop-2-yl radical then reacts with the tributyltin hydride to remove a hydrogen atom and creates a tributyltin radical.

The tributyltin radical then attacks the sulfur atom, initiating a chain or single electron transfers, and forms an alkyl radical.
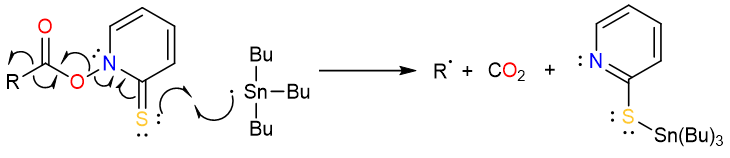
The alkyl radical then takes up a hydrogen atom from the tributyltin hydride to form the final alkane product.
Hunsdiecker Reaction
The Hunsdiecker reaction converts a silver salt of a carboxylic acid into an alkyl halide using bromine (Br2) in dry carbon tetrachloride (CCl4).
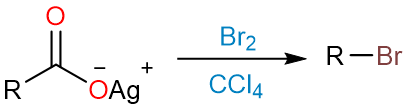
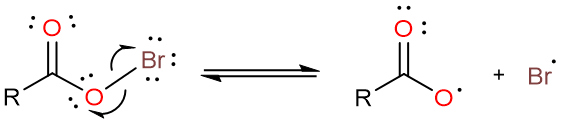
The reaction starts with the formation of an acyl hypobromite from silver carboxylate and bromine.
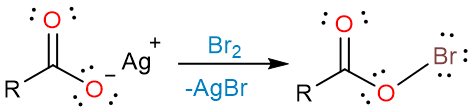
The weak O–Br bond is then homolytically cleaved to form bromine radical and a carboxylate radical.
The carboxylate radical undergoes the loss of a carbon dioxide molecule to form an alkyl radical.
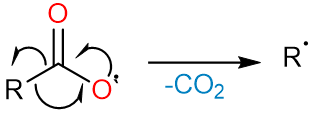
Finally, the combination of the alkyl radical with the bromine radical yields the alkyl bromide.
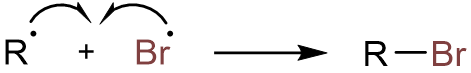
Kochi Reaction
This reaction is a variation of the original Hunsdiecker reaction. It converts a carboxylic acid into an alkyl chloride. The reaction takes place between a carboxylic acid and lithium chloride with lead(IV) acetate as the catalyst.

