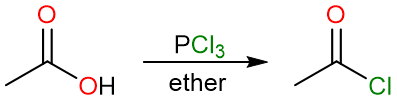
Conversion to Acyl Halides
An Introduction to Nucleophilic Acyl Substitution
The reactions of aldehydes and ketones are remarkably different from the reactions of carboxylic acids and their derivatives. Aldehydes and ketones give nucleophilic acyl addition reactions while carboxylic acids and derivatives give nucleophilic acyl substitution reactions.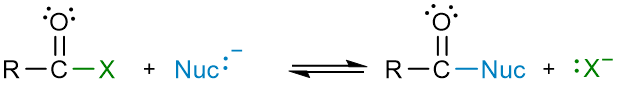
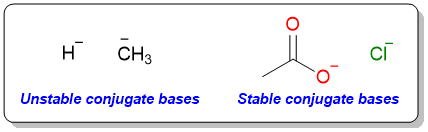
The reason behind this difference in reactivities is the type of leaving group attached to the carbonyl group. In aldehydes and ketones, the group bonded to the carbonyl group is either H or an alkyl/aryl group. These are the worst leaving groups because of two reasons: 1) The C–H and C–C bonds are very strong, and 2) The conjugate bases that would form as a result of bond cleavage – hydride anion (H−) or alkide anion (R−) – are very unstable to exist in solutions. On the other hand, carboxylic acids and their derivatives form relatively stable conjugate bases and therefore can react to give nucleophilic acyl substitution reaction.
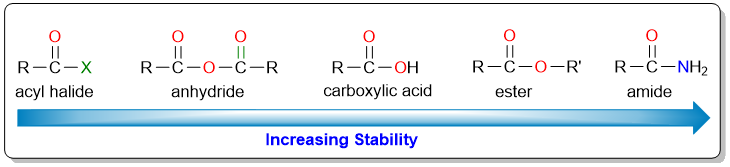
The reactivity of a carboxylic acid derivative towards nucleophilic acyl substitution reaction depends upon the stability of the conjugate base that would form as a result of the substitution reaction. Acyl halides and acid anhydrides are the most reactive because their conjugate bases (halide anion and carboxylate anion) are the most stable. On the other hand, amides are the least reactive (or the most stable) because their conjugate bases (amide anions) are the least stable.
Conversion of Carboxylic Acids to Acyl Halides
We will discuss the conversion of carboxylic acids to each of their derivatives one by one. This section is dedicated to the synthesis of acyl halides.
Synthesis of Acyl Halides using Thionyl Chloride
Carboxylic acids can be converted to acyl halides using either thionyl chloride (SOCl2), or oxalyl chloride ((COCl)2) under slightly basic conditions. A weak base, such as pyridine or triethylamine is often required to neutralize the acid (HCl) produced in this reaction.
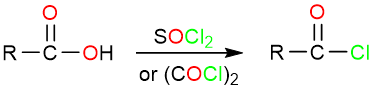
Reaction Mechanism
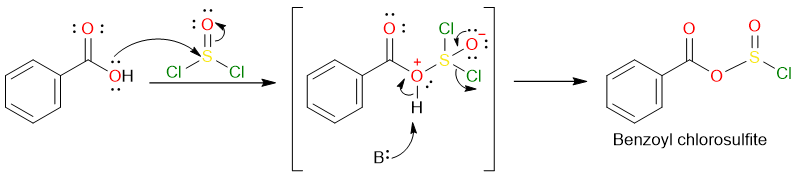
Some elements have great affinities for each other such as Si and F, O and P, and O and S. The conversion of a carboxylic acid into an acyl halide using thionyl chloride takes advantage of this fact. One of the products is SO2 in which one of the oxygen atoms comes from the carboxylic acid.
The reaction starts with the nucleophilic attack of the carboxylic acid hydroxy oxygen on the sulfur atom from the thionyl chloride. The base removed the proton from the charged intermediate and the subsequent loss of a chloride anion forms a chlorosulfite.
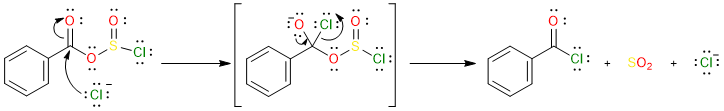
The chloride ion then attacks the carbonyl carbon of the chlorosulfite molecule, forming a tetrahedral intermediate which then collapses to give the acyl halide, SO2, and a chloride anion.
Conversion to Acyl Bromides using PBr3
Carboxylic acids, just like alcohols, can be very easily converted to alkyl bromides using phosphorous tribromide (PBr3). One mole of PBr3 can convert 3 moles of a carboxylic acid into acyl bromide.
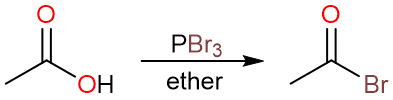
Note that acyl chlorides can also be prepared using phosphorous trichloride (PCl3).