Reduction of Carboxylic Acids
Reducing Carboxylic Acids
We saw in the synthesis of carboxylic acids section that carboxylic acids can be synthesized by the oxidation of primary (1°) alcohols and aldehydes. This means that the reduction of carboxylic acids should produce aldehydes and primary alcohols.
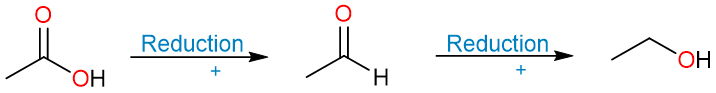
However, carboxylic acids are not reduced by weak reducing agents while strong reducing agents convert them into primary alcohols. This is because the reduction of a carboxylic acid produces an aldehyde first but that is never isolated because it is more easily reduced than the carboxylic acid itself. To get to the aldehyde as the final product, we have other approaches that we’ll get to later.
Reduction using LiAlH4
Lithium aluminum hydride, LiAlH4, is a very strong reducing agent and reduces carboxylic acids and all of their derivatives into primary alcohols. Except for amides, which are reduced to amines.
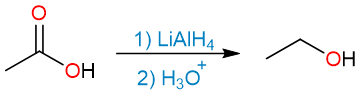
The mechanism of the reaction starts with an acid-base reaction between the acid and hydride reagent.
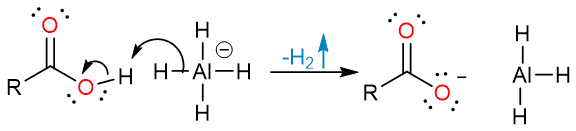
The carboxylate anion then attacks the aluminum atom of the AlH3. An internal hydride attack on the carbonyl carbon yields a tetrahedral intermediate which collapses to yield an aldehyde.
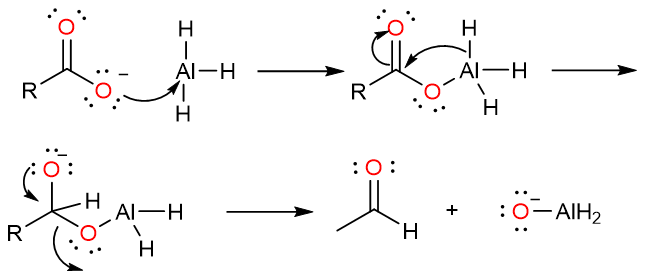
The aldehyde molecule is even more reactive than the initial carboxylic acid molecule, so it is attacked by the hydride reagent and an alkoxide is formed.
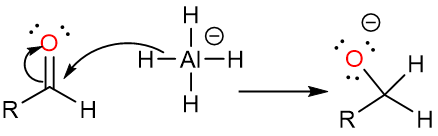
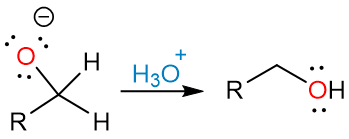
The aqueous acid workup step then destroys the remaining hydride reagent and forms the alcohol.
Diborane Reduction of Carboxylic Acids
One of the problems with lithium aluminum hydride is that it is not chemoselective. Chemoselectivity refers to the selective reactivity of a reagent for a specific functional group. Lithium aluminum hydride can reduce all types of carbonyl groups.
Let’s say we have a compound that has both a carboxylic acid and an ester functional group but only the reduction of the carboxylic acid group is desired. This transformation cannot be achieved by lithium aluminum hydride because it will reduce both groups to primary alcohols.
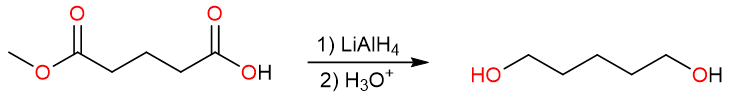
On the other hand, diborane (B2H6 or simply BH3 because B2H6 is a dimer) in tetrahydrofuran (THF) is the chemoselective reagent that could achieve this transformation. Diborane selectively reduces carboxylic acids in presence of esters.
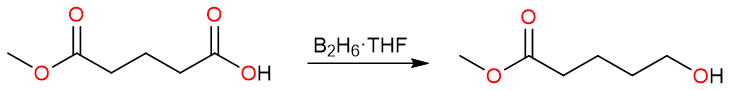
The mechanism of the reaction starts with the nucleophilic attack of the carbonyl oxygen on the boron atom forming a Lewis acid-base adduct.
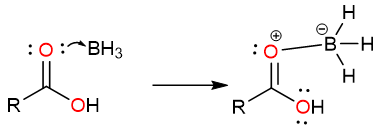
An internal hydride attack from the BH3 to the carbonyl carbon takes place and a tetrahedral intermediate is formed. The tetrahedral intermediate collapses to yield a protonated aldehyde.
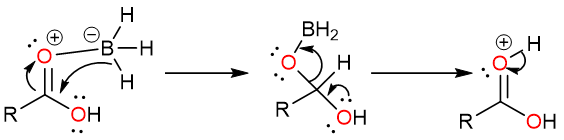
The aldehyde is very quickly deprotonated by the borate ion to yield a neutral aldehyde molecule.
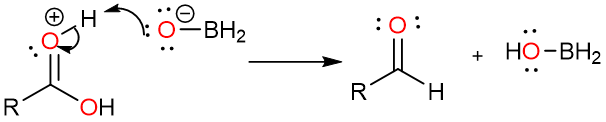
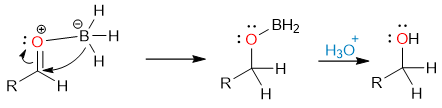
Now the aldehyde carbonyl oxygen attacks the boron atom again. Another Lewis acid-base adduct is formed.
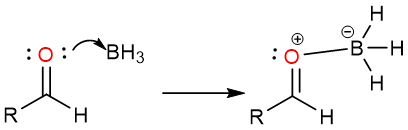
Another hydride attack forms the borate ester which is then hydrolyzed to alcohol by the addition of aqueous acid.
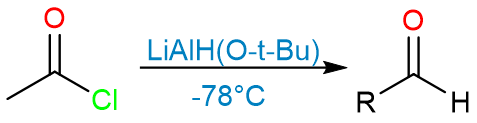
Reduction of Carboxylic Acids to Aldehydes
The reduction of carboxylic acids to aldehydes can be achieved by the reduction of carboxylic acid derivatives using sterically hindered hydride reagents such as lithium tri-siamylborohydride or lithium tri-ter-butoxyborohydride. These reagents are not very active due to steric hindrance and have only one hydride for reduction.
The reduction can be performed by first converting a carboxylic acid into an acyl halide either by thionyl chloride or phosphorous trichloride.
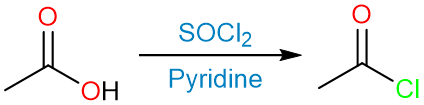
The reduction of the acyl halide using either lithium trisiamylborohydride or lithium tri-ter-butoxyborohydride gives the aldehyde.