Hell-Vollhard-Zolinsky Reaction
Hell-Vollhard-Zolinsky Reaction
An Introduction to α-Halogenation
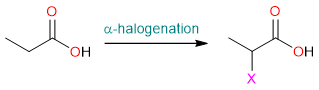
Alpha halogenation is a reaction that replaces a hydrogen atom on the carbon next to the carbonyl group (α-carbon) with a halogen atom.
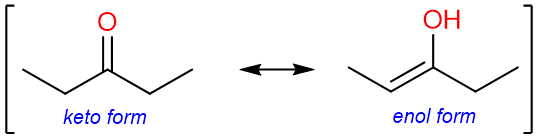
Carbonyl compounds tend to isomerize into their tautomers.
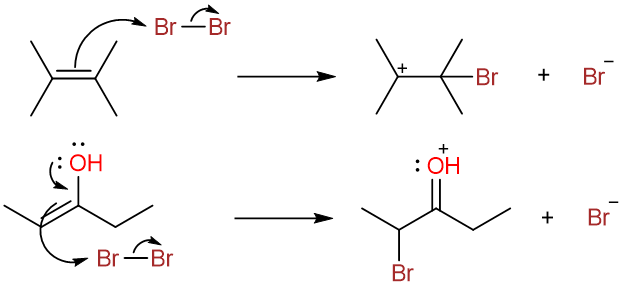
The reaction involves the nucleophilic attack of the enol double bond on the halogen molecule. The halogen molecule behaves as the electrophile in these reactions. In this way, these reactions are analogous to the halogenation of alkenes.
The Hell-Volhard-Zelinsky Reaction
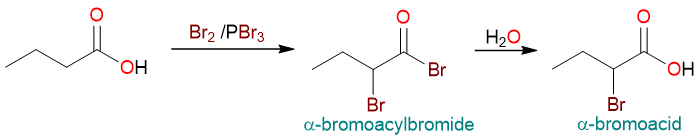
The Hell-Volhard-Zelinsky (HVZ) reaction is an excellent way to convert a carboxylic acid into an α-bromocarboxylic acid. α-bromocarboxylic acids are precursors to other useful molecules. For example, they can be converted to amino acids by a substitution reaction with an amine.
Mechanism of the Reaction
The HVZ reaction proceeds through an acyl bromide intermediate. This is because one of the reagents (PBr3) converts a carboxylic acid into an acyl bromide before the alpha bromination takes place.
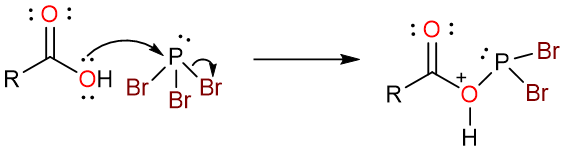
The reaction starts with the nucleophilic attack of the carbonyl hydroxy group on the phosphorous atom. This results in a loss of one bromide ion from the PBr3 molecule.
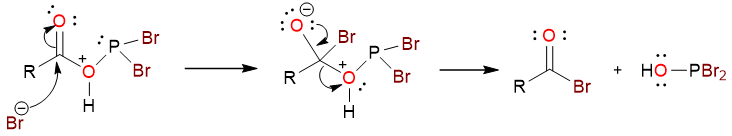
The bromide ion then attacks the carbonyl carbon to yield the tetrahedral intermediate which then collapses to yield the acyl bromide.
The acyl bromide then undergoes keto-enol tautomerism. The enol attacks the bromine molecule.
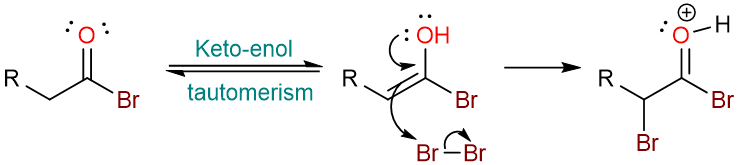
Deprotonation by the bromide ion yields the α-bromoacyl bromide.
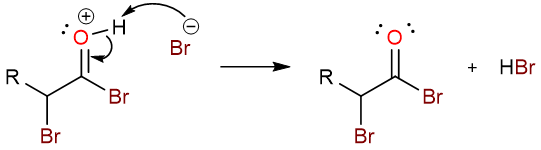
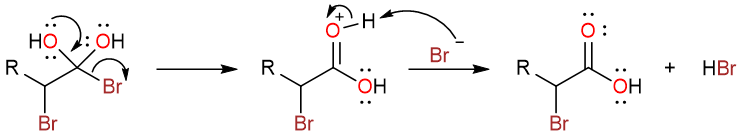
After the formation of the α-bromoacyl bromide, the second step of the reaction involves the reaction with water to give the α-bromocarboxylic acid.
This reaction is a usual nucleophilic acyl substitution reaction where a water molecule attacks the carbonyl carbon. The resulting tetrahedral intermediate undergoes a proton exchange to yield a geminal diol.
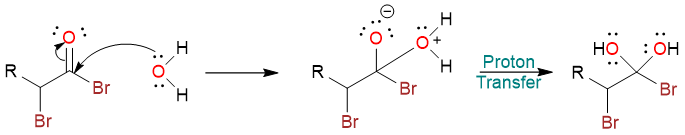
One of the hydroxy groups then pushes its lone pair onto the carbonyl carbon to expel the bromide ion and yields the protonated carboxylic acid. The protonated form then undergoes deprotonation to yield the carboxylic acid.