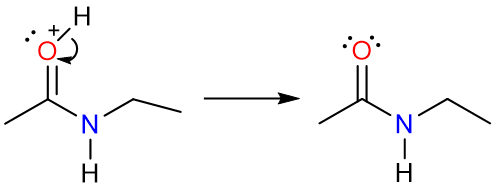
Conversion to Amide
Conversion to Amide
Amides are derivatives of carboxylic acids where the hydroxy group of a carboxylic acid has been replaced by a primary or secondary amine. Based on how many carbon atoms are directly attached to the nitrogen atom, amides can either be primary (1°) or secondary (2°), or tertiary (3°).
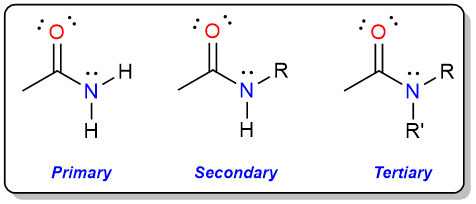
Amides are an important class of organic compounds. Proteins are made from amino acids held together by amide bonds (aka peptide bonds). Plastics such as Nylon, Kevlar, and Twaron are all amides.
Synthesis of Amides from Carboxylic Acids and Amines
One direct method for the preparation of amides is the reaction of a carboxylic acid with an amine.
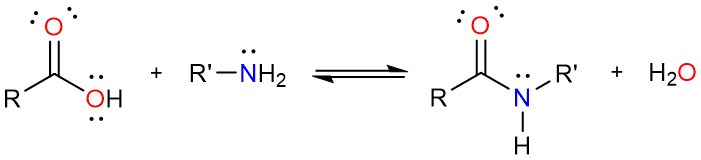
Straightforward as it may seem, this method is not without limitations. Carboxylic acids and amines react together to form a salt.
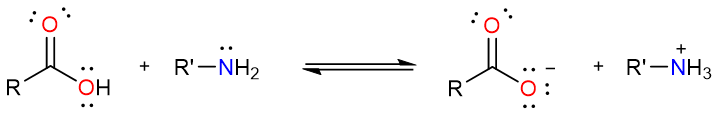
Since a carboxylate anion is a very weak electrophile and an ammonium ion is not nucleophilic at all, the reaction usually gives a salt instead of an amide. However, at higher temperatures (≥100 °C) that promote the evaporation of water molecules, the reaction is driven forward but the yields are not always impressive.
The use of some coupling agents such as the DCC (Dicyclohexyl carbodiimide) can improve the yields of this reaction to a great extent.
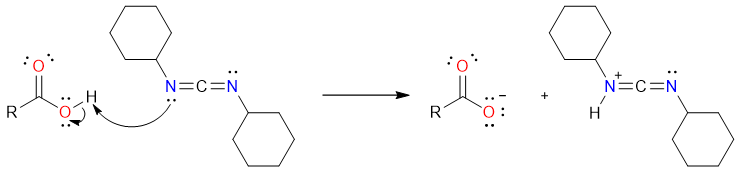
The reaction starts with the deprotonation of the carboxylic acid by one of the nitrogen atoms of the DCC molecule.
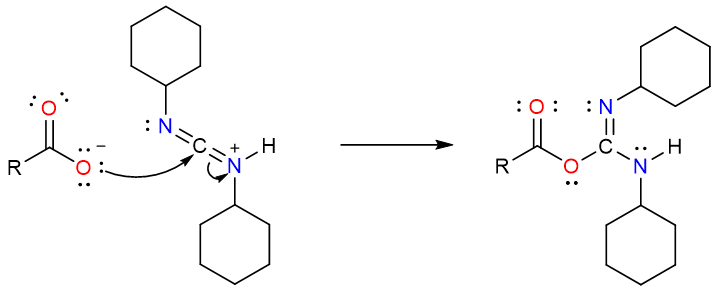
The carboxylate anion attacks the electrophilic carbon atom of the protonated DCC molecule.
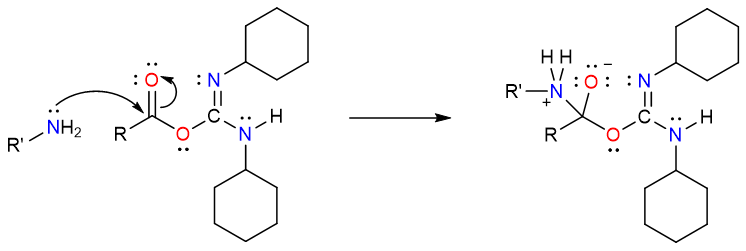
Due to the electron pull from the C=N bond, the carbonyl carbon atom is now sufficiently electrophilic to be attacked by the amine molecule.
An internal proton transfer takes place forming an excellent leaving group.
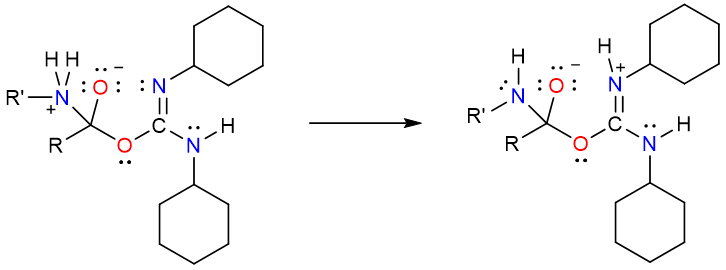
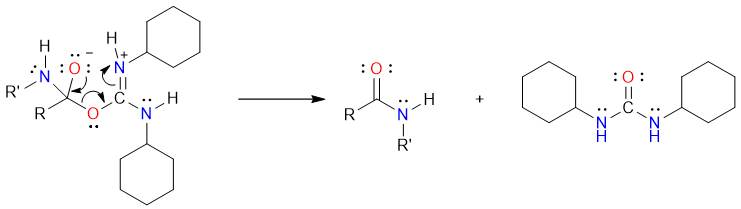
Finally, the electron push from the negatively charged oxygen atom results in the formation of an amide and a substituted urea byproduct (N,N’-dicyclohexylurea).
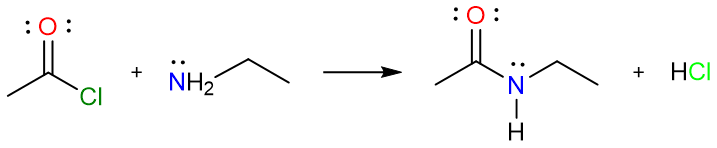
Synthesis of Amides for Acyl Halides and Acid Anhydrides
A great way to prepare amides in excellent yields is the reaction of an amine with an acyl halide or an acid anhydride. The reaction is driven forward due to the very large difference in stabilities of amides and acyl halides/acid anhydrides. This reaction always gives excellent yields.
Mechanism of the Reaction
Acyl halides and acid anhydrides are very unstable and even react with very weak nucleophiles. The reaction starts with the nucleophilic attack of the amine on the carbonyl carbon forming a tetrahedral intermediate that undergoes a proton transfer to yield its neutral counterpart.
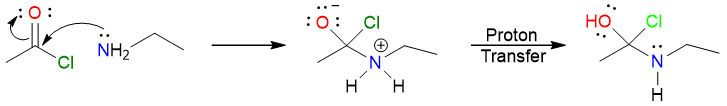
The electron push from the hydroxy group moves resulting in the chlorine atom leaving with a pair of electrons. A protonated amide is formed as a result.
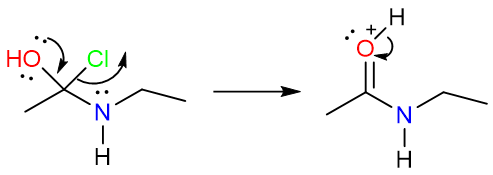
Finally, the loss of proton from the protonated amide yields the neutral amide.