Conversion to Nitrile
Introduction to Nitriles
Nitriles can be considered nitrogen analogs of terminal alkynes.
The carbon atom and the nitrogen atom are both sp hybridized. There is one sigma bond between carbon and nitrogen atoms formed from the overlap of two sp-hydridized orbitals. The rest of the two bonds in the triple bond are π-bonds which form from the overlap of unhybridized p orbitals on carbon and nitrogen atoms.
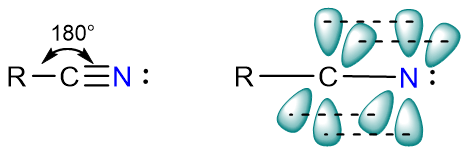
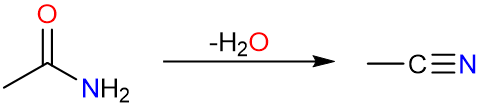
Synthesis of Nitriles from Carboxylic Acids
Nitriles can be synthesized by the dehydration of primary amides by SOCl2, POCl3, or P2O5.
Dehydration by SOCl2
Amide dehydration using SOCl2 takes place under slightly basic conditions. A weak base such as pyridine or triethylamine is used to neutralize the HCl produced in the reaction.
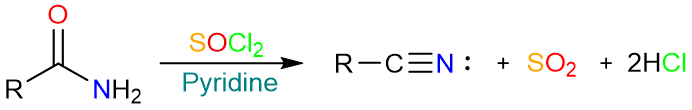
The mechanism of dehydration using thionyl chloride starts with the nucleophilic attack of the carbonyl oxygen on the sulfur atom of the thionyl chloride to form a tetrahedral intermediate. The tetrahedral intermediate collapses to form a chlorosulfite.
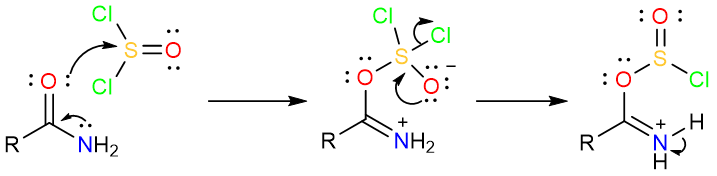
Subsequent removal of two protons from the chlorosulfite results in the expulsion of SO2 and the Cl− ion. The amide is formed as the product.
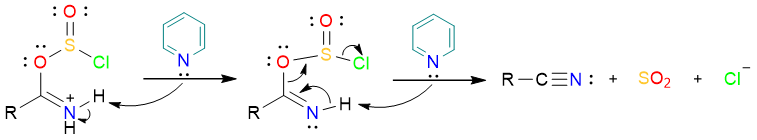
Dehydration by POCl3
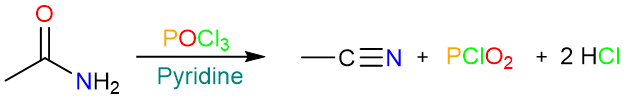
The dehydration of a primary amide by POCl3 follows a similar reaction mechanism. It starts with the nucleophilic attack of the carbonyl oxygen on the phosphorous atom. A tetrahedral intermediate is formed which then collapses to expel a chloride ion.
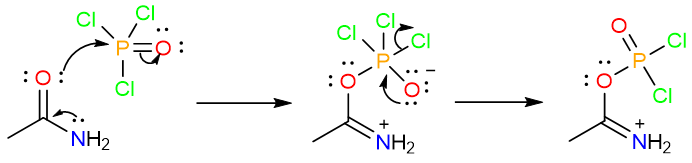
Then a series of two proton removals by the base yields the final product.
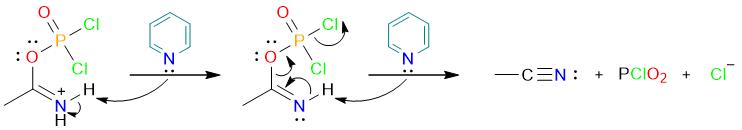
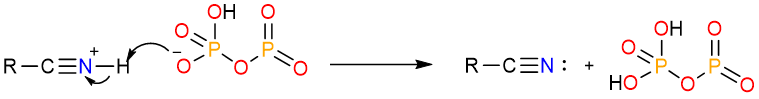
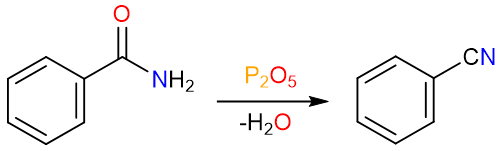
Dehydration by P2O5
Diphosphorus pentoxide is a very powerful dehydration agent. It converts primary amides to nitriles by a similar mechanism.
The reaction starts with the nucleophilic attack of the carbonyl atom on one of the phosphorous atoms. The formed tetrahedral intermediate undergoes an internal proton exchange.
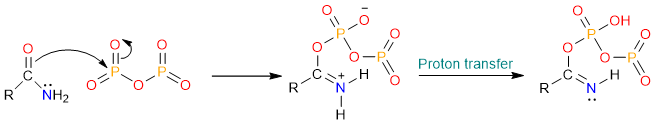
The nitrogen atom then pushes its lone pair onto the carbonyl carbon to expel the phosphorous leaving group.
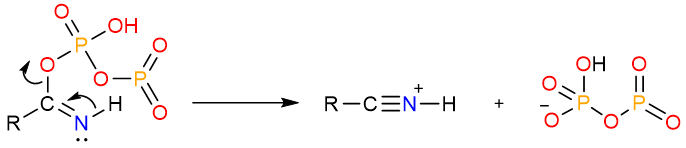
Finally, a deprotonation step yields the nitrile.