Acid-Base Principles
The acidity of Carboxylic Acids
Carboxylic acids are weak acids and they dissociate partially in the water.
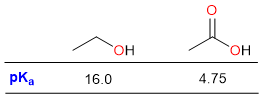
Although weak, carboxylic acids are significantly more acidic than other oxygen-containing compounds. For example, acetic acid is about 11 orders of magnitude stronger than ethanol.
The reason behind the acidity of carboxylic acids is the resonance stabilization of the carboxylate anion. As a general rule, the more stable the conjugate base, the stronger the acid.

The negative charge of the oxygen atom is spread over three atoms (one carbon and two oxygen atoms). The resonance hybrid has the following orbital structure:
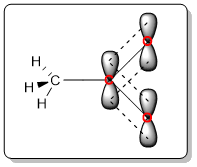
The carboxylate group has three unhybridized p orbitals that are aligned parallel to each other forming a path for the delocalization of the negative charge.
Effect of Substituents on the Acidic Strength of Carboxylic Acids
The acidity of carboxylic acids is strongly affected by the presence of substituents. The substituents affect the acidic strength through the inductive effect. Electron-donating groups (such as alkyl groups) decrease the acidity while electron-withdrawing groups (such as halogens) increase the acidic strength.
This is because electron-withdrawing groups pull the electron density away from the negatively charged region of the carboxylate anion and stabilize it. Electron donating groups increase the electron density on the carboxylate anion and destabilize it.
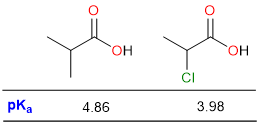
The effect of substituent on acidic strength can be seen in the two examples above. Due to the electron-withdrawing effect of the chloro group, the pKa of 3-chloropropanoic acid is much lower than the pKa of 3-methylpropanoic acid.
Effect of Substituent Electronegativity
In general, the acidity of carboxylic acid increases with the electronegativity of the substituent attached to the carboxylic acid. For example, in the four haloacetic acids below, the most acidic is fluoroacetic acid because fluorine is the most electronegative among all halogens while the weakest acid is the iodoacetic acid because iodine is the least electronegative.
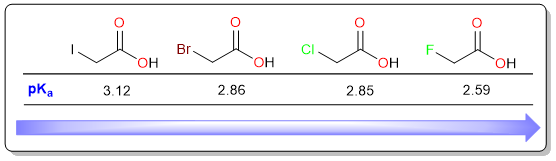
Effect of Substituent Distance
In addition to the electronegativity, the distance of the electronegative atom (or electron-withdrawing group) is also an important factor that determines the acidic strength. The closer the electron-withdrawing substituent to the carboxylic acid group, the higher the acidity.
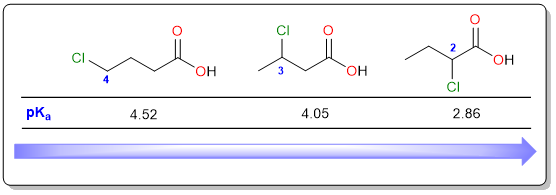
In the diagram above, the most acidic compound is 2-chlorobutanoic acid which is as acidic as chloroacetic acid. This is understandable because the distance between the chloro substituent and the carboxylic acid group is the same in both compounds. As the distance increases, the pKa value increases. 3-chlorobutanoic acid is less acidic than 2-chlorobutanoic acid. The least acidic is 4-chlorobutanoic acid which has a pKa value of 4.52 (very close to acetic acid, pKa 4.75). In general, the effect of a chloro substituent is almost nullified when it is more than three atoms away from the carboxylic acid.
Number of Electronegative Atoms
When there is more than one electronegative atom, the carboxylate anion is even more stabilized which makes the acid stronger.
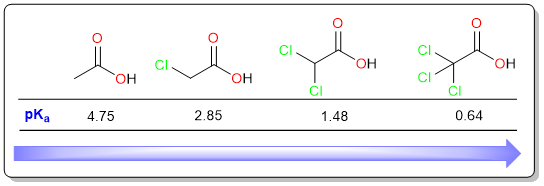
It can be seen in the examples above that the acidity increases as the number of chlorine atoms increases.
Substituent Effects in Benzoic Acids
Just like aliphatic acid, benzoic acids are also prone to substituent effects. Electron donating substituents decrease the acidic strength while electron-withdrawing substituents increase the acidic strength. It can be seen that 4-chlorobenzoic acid and 4-nitrobenzoic acid are both stronger than benzoic acid. On the other hand, methyl and methoxy substituents decrease the acidic strength of benzoic acid.
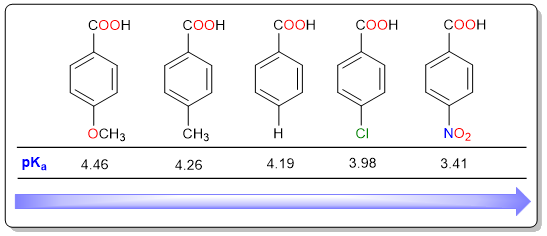
Resonance Vs Inductive Effect
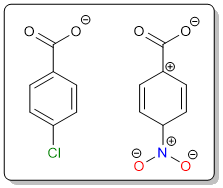
When it comes to benzoic acids, resonance affects acidity more than the inductive effect. This is evident from the differences in pKa values between 4-chlorobenzoic acid and 4-nitrobenzoic acid. Although the electronegativities of nitrogen and chlorine as the same, 4-nitrobenzoic acid is a stronger acid because the nitro group can stabilize the carboxylate anion through resonance while the chloro group can only pull electron density through its inductive effect.
Hydrogen Bonding in o-Substituted Benzoic Acids
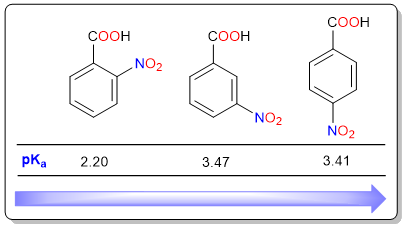
Nitrobenzoic acids are a special case because their acidities cannot be explained solely based on the distance of the nitro group from the carboxylic acid group. It can be seen that there is only a very slight difference in the acidic strengths of m-nitrobenzoic acid (3-nitrobenzoic acid) and p-nitrobenzoic acid (4-nitrobenzoic acid). On the other hand, o-nitrobenzoic acid (2-nitrobenzoic acid is a much stronger acid.
The reason behind this exceptional difference is not just the distance, it is the hydrogen bond that forms between the hydroxy group of the carboxylic acid and the negatively charged oxygen atom of the nitro group.
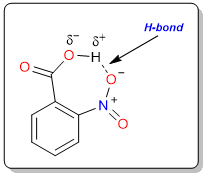
The hydrogen bond between the H and O of the nitro group weakens the bond between O and H of the hydroxy group. Due to the weak bond, the dissociation of the acid becomes easier and this leads to increased acidity.
