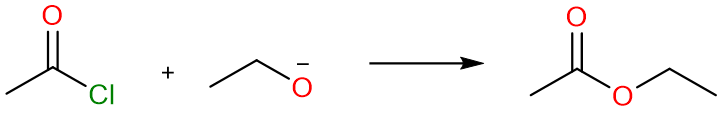Conversion to Ester
An Introduction to Esters
Esters are derivatives of carboxylic acids where the hydroxy group of the carboxylic acid has been replaced by an alkoxy group. Esters are widespread in various forms. For example, fats and oils are high-molecular-weight esters formed from trihydroxy alcohol (glycerol or glycerine) with a long-chain carboxylic acid (a fatty acid). Waxes are monoesters formed by the condensation of a long-chain alcohol (fatty alcohol) with a fatty acid.
Low-molecular-weight esters are principal components responsible for the taste and fragrance of many fruits and essential oils. For example, amyl acetate is responsible for the characteristic smell of bananas and octyl acetate is responsible for the aroma of oranges. Linalyl acetate is the principal component of the lavender and sage essential oil while methyl salicylate is found in the oil of wintergreen.
Synthesis of Esters: Fischer Esterification
One of the earliest methods devised in laboratories for the synthesis of esters is the direct reaction of a carboxylic acid with an alcohol in presence of a catalytic amount of sulfuric acid – The Fischer esterification reaction.
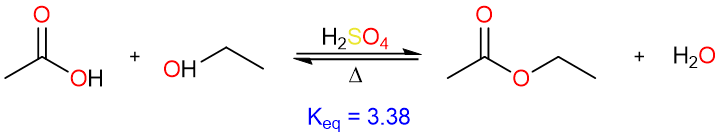
The formation of the ester is however reversible and the yields are usually not great.
Mechanism of Fischer Esterification
The reaction starts with the protonation of the carbonyl oxygen. This increases the electrophilicity of the carbonyl carbon.
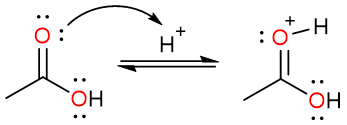
The alcohol oxygen then attacks the carbonyl carbon to produce a tetrahedral intermediate which undergoes a proton loss to yield a geminal diol.
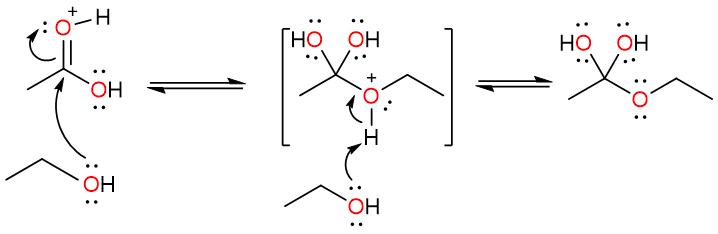
Another protonation converts one of the hydroxy groups of the diol into the water molecule – which is an excellent leaving group. The electron push from the unprotonated hydroxy group results in the loss of a water molecule, forming a protonated ester.
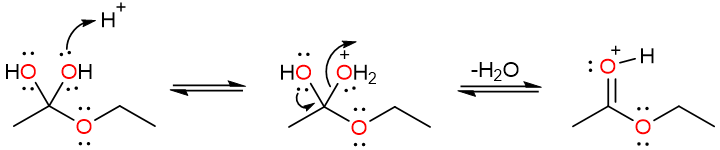
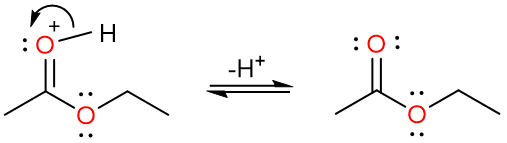
Finally, the loss of a proton results yields the ester. However, it is important to note that every step of this reaction is reversible and that is why this reaction usually does not give high yields of esters.
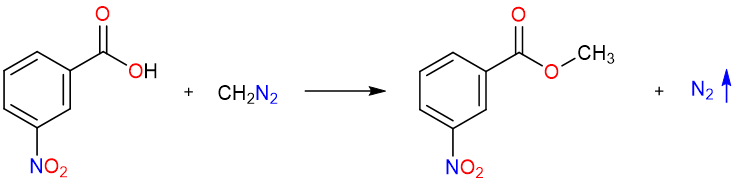
Ester Synthesis using Diazomethane
An excellent method for the formation of methyl esters is the reaction of a carboxylic acid with diazomethane. The reaction gives high yields but has limited applicability because only methyl esters can be prepared. Furthermore, diazomethane is explosive and a safety hazard in large quantities, so its applications are only within laboratories.
Mechanism of the Reaction
Diazomethane has the following two resonance forms:
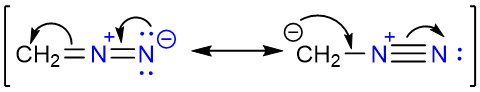
As can be seen that one of the resonance forms has a nucleophilic carbon atom. The reaction starts with the removal of the carboxylic acid proton by the negatively charged carbon atom of the diazomethane.
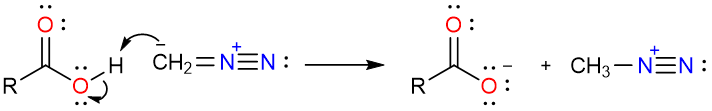
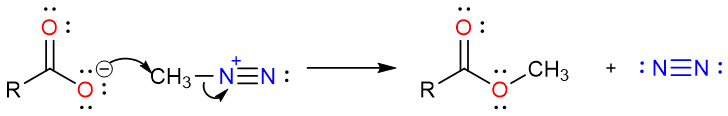
Since nitrogen gas is an excellent leaving group, its loss will result in the formation of methyl carbocation which is too stable to exist. This results in the highly electrophilic nature of the methyl group in the protonated diazomethane molecule. The negatively charged oxygen atom of the acetate ion attacks the methyl group pushing the nitrogen molecule away and producing methyl acetate. One of the reasons this reaction gives high yields is the loss of a nitrogen molecule as gas. Since one of the products is continuously removed from the reaction mixture, the reaction is forced in the direction of the products (Le Chatelier’s Principle).
Ester Synthesis using Acyl Halides and Acid Anhydrides
We know that acid halides and acid anhydrides are too electrophilic and unstable and are readily attacked by even the weakest nucleophiles. This presents a versatile method for the synthesis of esters in high yields.
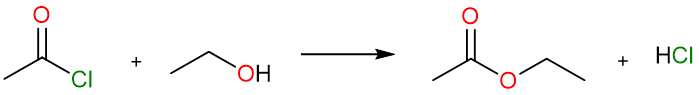
The reaction can take place using either alcohol or an alkoxide. Alkoxides, being stronger nucleophiles react faster (than alcohols) with acid halides and acid anhydrides.