Properties of Carboxylic Acids
An Introduction to Carboxylic Acids
Carboxylic acids are an important class of carbonyl compounds. This class of compounds is very commonly found in nature. For example, formic acid is found in ant and bee stings and acetic acid is the chief component of vinegar. Oils, fats, and waxes are derivatives (esters) of long-chain carboxylic acids (fatty acids). Soaps are carboxylic acid salts made from the basic hydrolysis (saponification) of oils and fats.
Structure of Carboxylic Acids
Carboxylic acids are structurally related to aldehydes and ketones in a way that they share the carbonyl group. The carbonyl carbon is sp2-hybridized and planar. One side of the carbonyl group is attached to a hydroxy group (–OH) while the other is bonded to an alkyl or aryl group. The chemical structure of formic acid along with bond angles and bond lengths is given below:
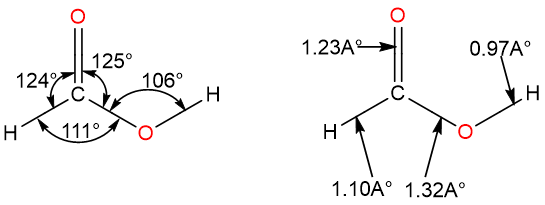
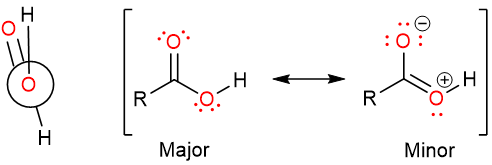
It can be seen that the bond angles around the carbonyl carbon are not symmetrical. This is due to the lone pair-bond pair repulsion: The lone pair on the carbonyl oxygen atom repels the hydroxy group and the hydrogen. It is interesting to note that carboxylic acids are most stable in their eclipsed conformation. The stability of the eclipsed conformation is due to the delocalization of one lone pair of hydroxy oxygen over the carbonyl group pi system.
Physical Properties of Carboxylic Acids
Boiling Points
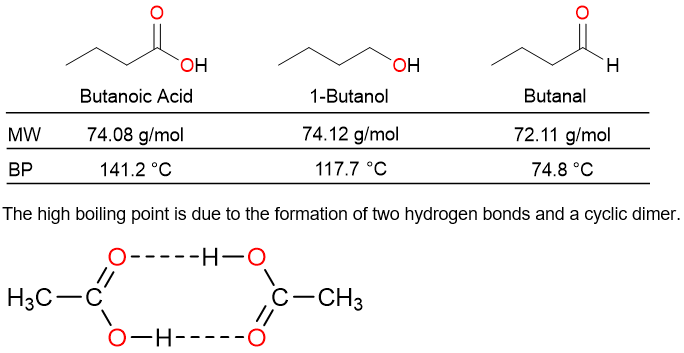
When compared with alcohols and aldehydes of similar molecular weights, the boiling points of carboxylic acids are quite high. for example, propanoic acid (MW = 74.08 g/mol) boils at 141.2 °C while 1-butanol (MW = 74.12 g/mol) boils at 117.7 °C and butanal (MW = 72.11 g/mol) boils at 74.8 °C.
Melting Points
The melting points of carboxylic acids depend upon a couple of factors in addition to the strength of hydrogen bonds. These factors include chain length and the presence of double bonds (especially cis bonds). Carboxylic acids that contain more than eight carbon atoms are usually solids at room temperature except if they contain C=C double bonds. As a general rule, the longer the carbon chain the higher the melting point. For example, octanoic acid (C7H15COOH) melts at 16 °C while decanoic acid (C9H19COOH) melts at 31 °C. carboxylic acids with cis double bonds have very low melting points. This is because the carbon chain can never stay straight and the formation of a stable crystal lattice is not possible. For example, stearic acid and linoleic acid both contain 18 carbon atoms except the former does not have any C=C double bonds while the latter has two cis double bonds. Stearic acid melts at 70 °C while linoleic acid melts at −5 °C
Similarly, dicarboxylic acids (such as succinic acid) have higher melting points. This is because there are two carboxylic acid groups in a single molecule and this results in a higher number of hydrogen bonds.
Solubilities of Carboxylic Acids
Carboxylic acids can form hydrogen bonds. This means they should be soluble in polar solvents. Small carboxylic acids (up to butanoic acid) are completely miscible with water. However, as the chain length of a carboxylic acid increases, its nonpolar nature increases, and its solubility in water decreases as a result. When the chain length reaches ten, the solubility in water becomes nearly zero. However, carboxylic acids show excellent solubilities in alcohols because of two reasons: First, alcohols can form hydrogen bonds and second alcohols are less polar and their nonpolar chain part can solvate the nonpolar carbon chain of the carboxylic acid through van der Waals interactions.
