Nomenclature of Carboxylic Acids
Common Names of Carboxylic Acids
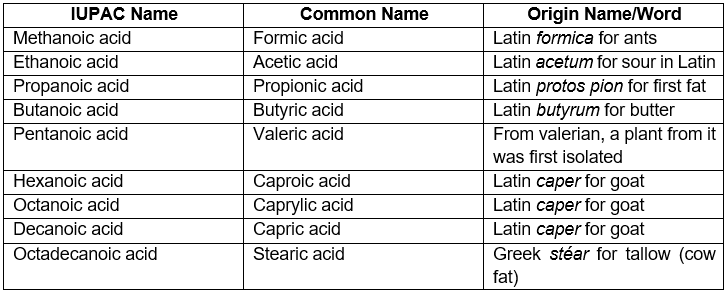
Carboxylic acids are among the first organic compounds to be isolated from various natural sources. They have been known for too long and therefore many of them have common names. Many of the common names we know today, come from some Latin words. For example, formic acid comes from the Latin formica for ants because it was first extracted from ants. Similarly, the name acetic acid comes from the Latin acetum (sour). Another example is butyric acid which is responsible for the smell and taste of rancid butter. The name comes from butyrum which is Latin for butter.
The following table shows the common names of a few common carboxylic acids along with the Latin words which the names come from.
IUPAC Names for Carboxylic Acid
The IUPAC nomenclature of carboxylic acids uses the root name of the alkane that corresponds to the longest and continuous chain of carbon atoms. The name is written as “alkanoic acid”.
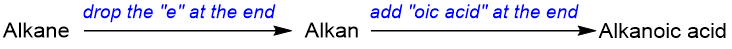
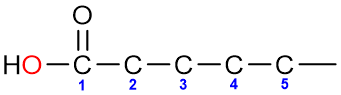
The numbering of the chain starts from the carbonyl carbon itself and goes on until the end of the chain.
The substituents get their numbers as the chain is numbered. For example:
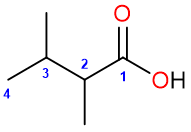
The substituents get their numbers as the chain is numbered. For example:
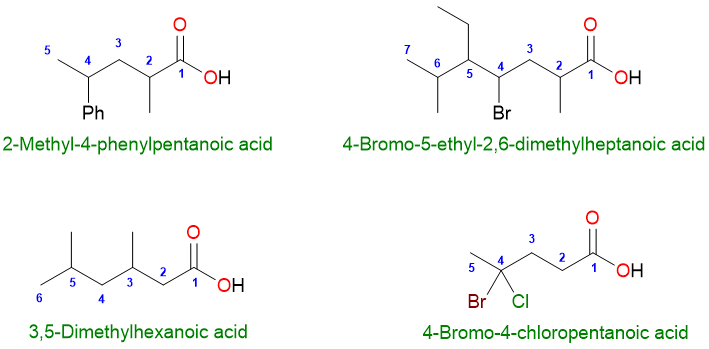
The carbonyl group takes priority over all functional groups except other carbonyl derivatives such as esters, amides, acyl halides, etc. Functional groups such as alcohols, aldehydes, and ketones are written as their prefixes such as hydroxy and oxo.
Two Ways to Write Carboxylic Acid Names with Double Bonds
When there are double bonds in a carboxylic acid structure, the name of that acid can be written in two ways. For example:
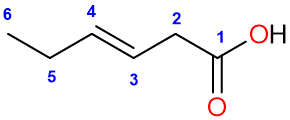
This compound has a trans or E double bond at position 3. We can either add the position of the double bond in the name at the start of the root name (3-hexene) or the middle, before the “ene” suffix (hex-3-ene).
The name of the compound is:
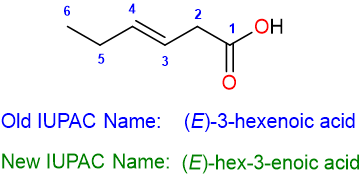
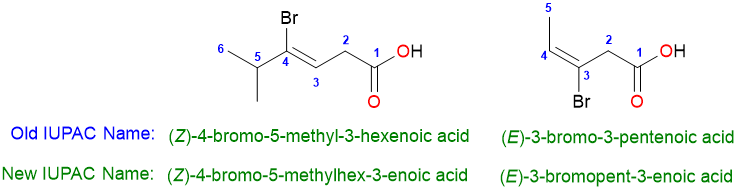
A couple more examples of the use of this method of nomenclature are:
Cyclic Carboxylic Acids
When the carboxylic acid functional group is directly attached to a cycloalkane ring, the suffix used for nomenclature is “carboxylic acid” instead of “oic acid”. The numbering starts from the carbon that has the -COOH group attached and goes on to cover the ring in the direction which covers the nearest substituent first.

The first compound here has the carboxylic acid at position 1 and the numbering goes clockwise to cover the bromo substituent because it's nearer than the chloro substituent.
In case there are two substituents on the ring that are at the same distance from the carboxylic acid (position 1), the numbering goes in the direction which covers the substituent with the alphabetical preference. For example:
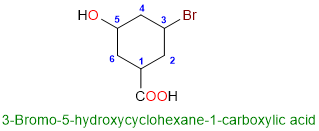
In the compound above, the distance from the carboxylic acid to either the bromo group or the hydroxy group is the same. However, we numbered the ring in the anticlockwise direction because that covers the bromo group first. This is because the bromo group takes preference over the hydroxy group. After all, its name starts with “B” while the hydroxy starts with “H”.
Compounds with Ring Substituents
Finally, let’s discuss some nomenclature with ring substituents. When the chain contains the carboxylic acid group, the ring attached to the chain becomes the substituent. There is nothing too difficult to remember about the names of these compounds except that the benzene ring is written as the phenyl group. The names of cycloalkane substituents appear as “cycloalkyl”.
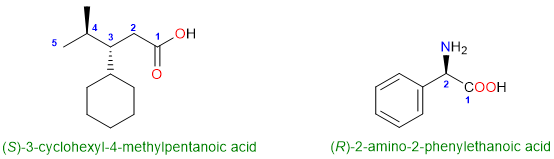
In the second compound, the chain is only two carbon atoms long, so it gets the name ethanoic acid (which can also be named acetic acid). The benzene ring at position 2 is named 2-phenyl.
