Conversion to Acid Anhydride
Introduction to Carboxylic Acid Anhydrides
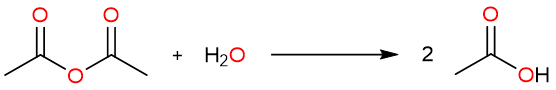
Carboxylic acid anhydrides are very unstable derivatives of carboxylic acids. They are strongly electrophilic and react with even the weakest nucleophiles. For example, acetic anhydride reacts readily with water forming acetic acid.
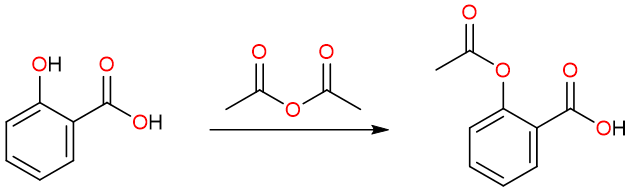
Anhydrides are important reagents to transfer acyl substituents to O and N atoms in various molecules. For example, the synthesis of aspirin involves the acetylation of salicylic acid.
Synthesis of Acid Anhydrides
Since acid anhydrides are very unstable, there aren’t many synthetic approaches to prepare them. We’ll discuss a couple here.
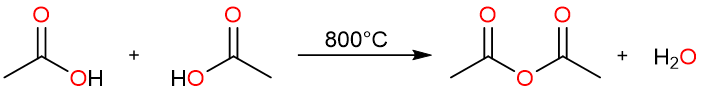
Dehydration of Carboxylic Acids
Acetic anhydride can be prepared by heating acetic acid at 800 °C.
Dicarboxylic acids on the other hand react under very mild conditions and form cyclic anhydrides. The following reaction shows the formation of succinic anhydride from succinic acid.
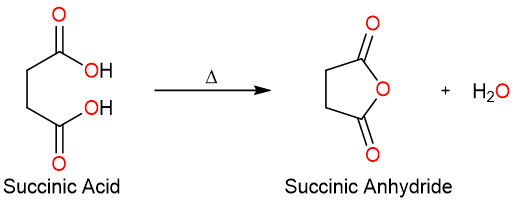
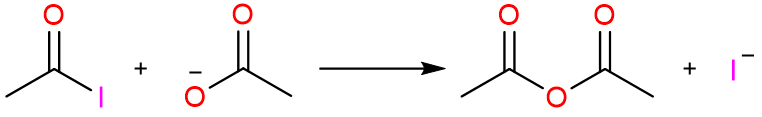
The reaction of Acyl Halides with Carboxylate Salts
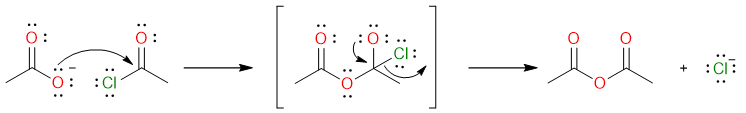
This is the most straightforward method for the synthesis of an acid anhydride and gives a good yield of products. The following reaction shows the reaction of acetyl chloride with sodium acetate to give acetic anhydride.

The reaction mechanism is quite simple. It starts with the nucleophilic attack of acetate ion on the acetyl halide molecule to form a tetrahedral intermediate which collapses to yield acetic anhydride.
Tennessee Eastman Acetic Anhydride Process
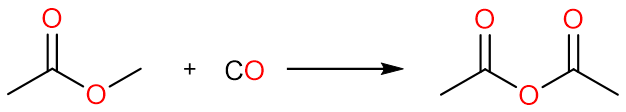
An excellent industrial process for the production of acetic anhydride is the carbonylation of methyl acetate to yield acetic anhydride. The reaction is carried out under anhydrous conditions using a rhodium(III) chloride catalyst.
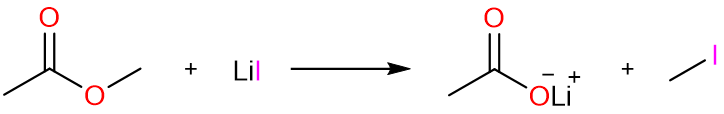
The reaction starts with the reaction of methyl acetate with lithium iodide to give methyl iodide and lithium acetate.
The carbonylation of methyl iodide with carbon monoxide yields acetyl iodide.
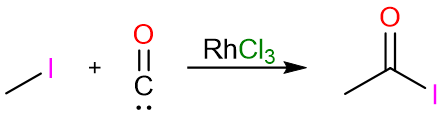
The nucleophilic attack of the acetate anion on the acetyl iodide molecules finally yields acetic anhydride.