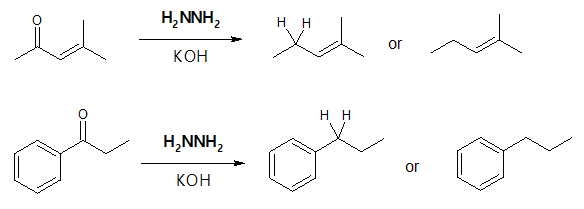
Wolff Kishner Reduction
WOLFF-KISHNER REACTION
Aside from imine and enamine formation, another interesting reaction of an aldehyde or a ketone with a nitrogen-containing compound is the Wolff-Kishner reaction. If you look at the overall reaction, this named reaction is a useful method of converting aldehydes and ketones into an alkane.
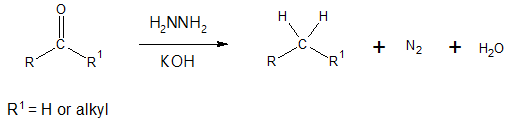
The above reaction is carried out by treating the aldehyde or ketone with hydrazine, H2NNH2, in the presence of potassium hydroxide, KOH. The reaction may look very simple as the oxygen double bond is would seem to be just replaced by two hydrogen atoms, converting the starting carbonyl compound to saturated hydrocarbon or alkane, with the production of nitrogen gas (N2) and water (H2O). But to see what is really happening in between the starting aldehyde or ketone and the alkane product, let’s examine the mechanism involved for the Wolff-Kishner reduction which will be illustrated shortly.
To give you an overview of the transformations involved, the first few steps will yield an intermediate called hydrazone, R2C=NNH2. It is then followed by base-catalyzed double bond migration, loss of nitogen (N2) gas, and finally, protonation resulting to the alkane product.
What induces the carbon-nitrogen double bond (C=N) of the hydrazone intermediate to migrate? It is when the base catalyst, usually hydroxide ion (OH-), removes one of the weakly acidic NH protons where a hydrazone anion is produced. By resonance, the double bond shifts from C=N, forming a new N=N of the hydrazone anion. The carbon is then reprotonated to generate the double-bond rearrangement product. It is then followed by thermodynamically favoured loss of nitrogen (N2) molecule and corresponding formation of another alkyl anion.
Wolff-Kishner reduction has a similar outcome as the catalytic hydrogenation of acylbenzene, converting the acyl group to an alkyl group. The advantage of Wolff-Kishner method is that it can be applied to both alkyl and aryl ketones.
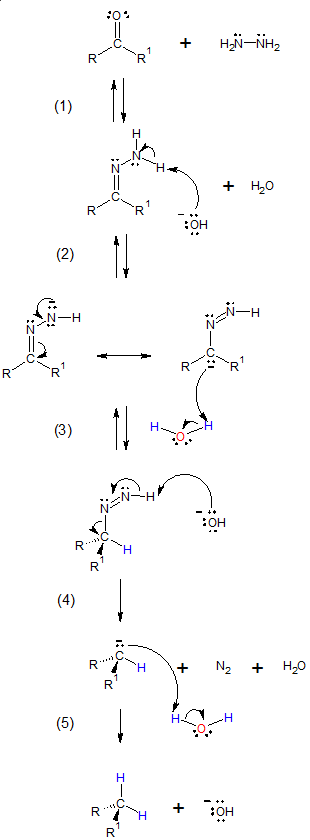
Mechanism of Wolff-Kishner reduction:
(1) Hydrazone is formed from the reaction of aldehyde or ketone with hydrazine.
(2) Base abstracts a weakly acidic N-H proton, forming a hydrazone anion. By resonance, the carbon carries a negative charge and the double bond between nitrogens is formed.
(3) The negatively charged carbon of the hydrazone ion is protonated to yield a neutral intermediate.
(4) The remaining weakly acidic N-H proton is abstracted again by the base with simulataneous release of N2, resulting to a carbanion.
(5) The carbanion formed is then protonated with the presence of H2O, giving the alkane product.
Additional sample reactions: