Tollens Test
TOLLEN’S TEST
Aldehydes can be easily oxidized to produce carboxylic acids compared to ketones. A lot of reagents can be used such as bleach (sodium hypochlorite, NaHClO), chromic acid (H2CrO4), and permanganate (MnO4-).
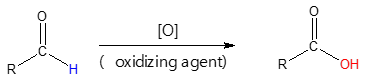
Even mild reagents such as silver oxide, Ag2O, can selectively oxidize aldehydes in the presence of other functional groups. In addition, the use of Ag2O in aqueous ammonia makes it an ideal pathway in oxidizing acid-sensitive aldehydes, which could undergo unwanted side reactions when done in acidic environment such as CrO3 oxidation. The said reagent is also known as Tollen’s reagent, and now commonly used to distinguish aldehydes from ketones in a test referred to as Tollen’s test or silver-mirror test. You will see later why it is called as such.
Tollen’s reagent is a colorless, basic, aqueous solution containing silver ions complexed with ammonia, NH3, [Ag(NH3)2+]. Two steps are involved in preparing this reagent:
First step: Aqueous solution of silver nitrate (AgNO3) is mixed with aqueous sodium hydroxide (NaOH)
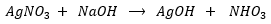
Silver hydroxide then decomposes to silver oxide and water:
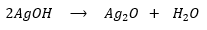
Silver oxide, Ag2O precipitates out of the solution.
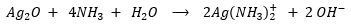
How can Tollen’s reagent bring about the oxidation of aldehydes to their corresponding carboxylic acids? Shown below is the general reaction of the oxidation process.
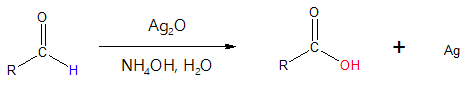
Notice that the silver ion (Ag+) in silver oxide serves as the oxidizing agent and if you recall, oxidizing agents are reduced in the process. Silver ion, in this case, is reduced to silver metal (Ag), which is deposited in the reaction mixture. As the reaction proceeds, the silver metal that is formed is observed as a “silver mirror”, since you can actually see yourself when you look at the wall of the flask containing the reaction mixture surrounded with silver metal.
If you have two unknown compounds, one as an aldehydes and the other as a ketone, you can identify which is which by conducting Tollen’s test since only aldehydes respond to the said test while ketones will show negative result (absence of silver mirror).
The ionic equation of the oxidation process is shown below:
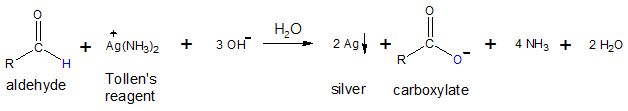
Notice that the silver ion (Ag+) in silver oxide serves as the oxidizing agent and if you recall, oxidizing agents are reduced in the process. Silver ion, in this case, is reduced to silver metal (Ag), which is deposited in the reaction mixture. As the reaction proceeds, the silver metal that is formed is observed as a “silver mirror”, since you can actually see yourself when you look at the wall of the flask containing the reaction mixture surrounded with silver metal.
If you have two unknown compounds, one as an aldehydes and the other as a ketone, you can identify which is which by conducting Tollen’s test since only aldehydes respond to the said test while ketones will show negative result (absence of silver mirror).
The ionic equation of the oxidation process is shown below:
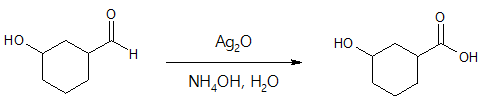
Example 2:
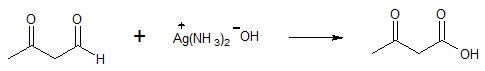
The above examples illustrate the specificity of Tollen’s reagent in oxidizing aldehyde group (-CHO) into carboxylic acid (-COOH) even when other functional groups are present in the compound being oxidized.
