Reduction of Aldehydes and Ketones
Reduction of Aldehydes and Ketones
Reduction of aldehydes and ketones can be done by nucleophilic addition of hydrides to aldehydes and ketones. This reaction converts aldehydes or ketones to alcohols. The common reagents used include lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4).
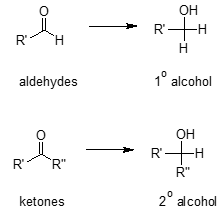
Generally, aldehydes produced primary alcohols while ketones give secondary alcohols.
General Mechanism of Reduction Reaction of Aldehydes and Ketones
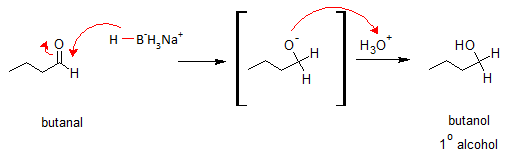
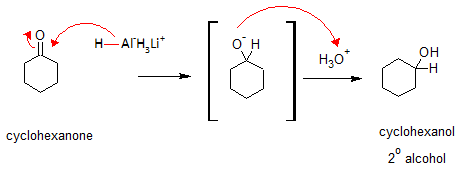
The nucleophilic H in the hydride reagent adds to the electrophilic C in the polar carbonyl group in the aldehyde, electrons will be drawn towards the electronegative oxygen in the carbonyl group (C=O) creating an intermediate metal alkoxide complex. Lastly, protonation of the alkoxide oxygen creates the primary alcohol product from the intermediate complex. Same mechanism is followed by ketones. They only differ in the type of alcohol product formed.

For example, reduction of cyclohexanone with Lithium aluminum Hydride would give Cyclohexanol. Here’s how the reaction goes. Following the same reaction mechanism, the reaction would give a secondary alcohol.
Given butanal (an aldehyde), reduction reaction with NaBH4 would give butanol, a primary alcohol.