Grignard Addition to Aldehydes and Ketones
Grignard Addition to Aldehydes and Ketones
Aldehydes and ketones generally reacts with Grignard reagent and undergo nucleophilic addition reaction to form alcohols. Grignard reagents are organometallic compounds with a formula RMgX. They are formed by covalent bonds between carbon atoms and metal atoms as shown in (1). In the laboratory, Grignard reagents are formed by refluxing alkylhalide/haloalkanes (dissolved in diethyl ether) with magnesium metal as shown in (2). Organometallic reagents are useful because they have nucleophilic carbon atoms.
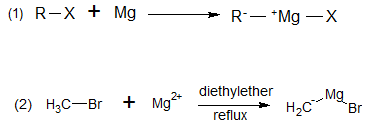
Grignard reagents may be made from primary, secondary, and tertiary alkyl halides, as well as from vinyl and aryl halides. Alkyl iodides are the most reactive halides, followed by bromides and chlorides. Alkyl fluorides generally do not react.
General Mechanism of Addition of Grignard Reagents
The mechanism for nucleophilic addition of Grignard reagents to aldehydes and ketones are as follows. The carbonyl functional (C=O) group will form a complex with Mg2+, followed by a nucleophilic addition of R: - to form an alkoxide intermediate and then protonation by dilute acid or water to yield the neutral alcohol. Moreover, Grignard additions are irreversible because the nucleophile, a carbanion, is not a leaving group.
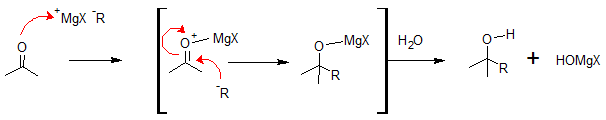
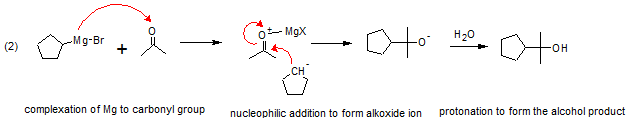
Take for example addition of Bromocyclopentane to acetone would produce 2-cyclopentyl-2-propanol.
The following shows how it’s done. First bromocycopentane would react with Mg to form cyclopentyl magnesium bromide as shown in (1).
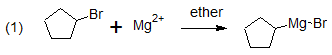
Then there would be complexation of Grignard reagent to acetone. This will be followed by nucleophilic addition of cyclopentyl carbanion to acetone to give alkoxide ion followed by protonation to give 2-cyclopentyl-2-propanol.
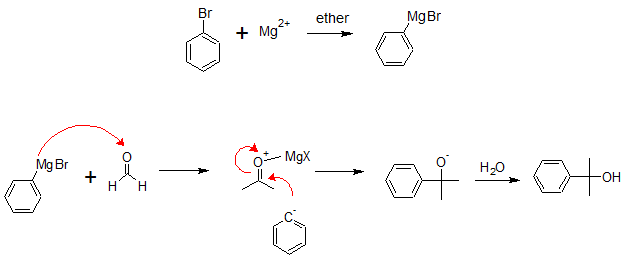
Similarly, addition of phenylbromide to formaldehyde would also follow the same reaction mechanism to give 2-phenyl-2-propanol.