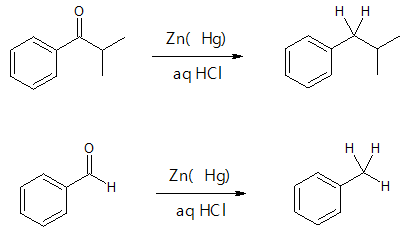
Clemmensen Reduction
CLEMMENSEN REDUCTION OF ALDEHYDES AND KETONES TO ALKANES
Another method of reducing aldehydes and ketones to alkanes is through Clemmensen reduction. It is especially useful when the alkylbenzene you want to synthesize is difficult to produce from direct Friedel-Crafts alkylation. From acylbenzene, Clemmensen reduction is done to yield the corresponding alkyl benzene by treatment with aqueous HCl and amalgamated zinc (zinc treated with mercury salts). The reaction was names after Danish chemist Erik Christian Clemmensen.
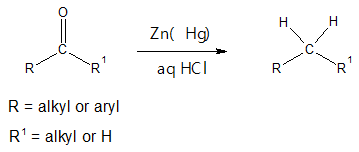
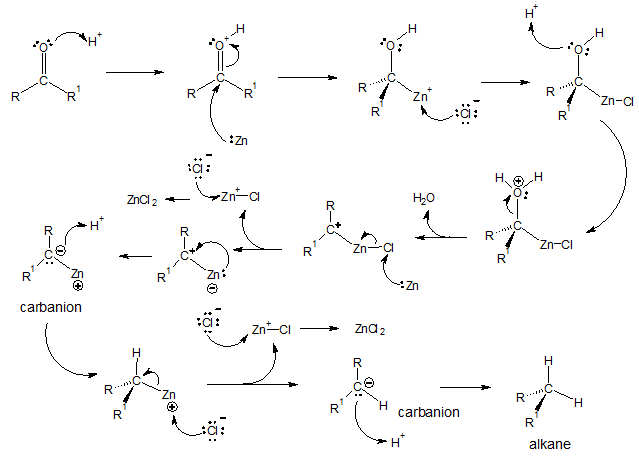
The above reaction is seldom used to reduce aliphatic ketones to alkanes but it is more effective in reducing aryl ketones to alkylbenzenes. The mechanism for Clemmensen reduction is not yet fully understood but there are two popular proposed reaction pathways, “Carbanionic mechanism” and “Carbenoid mechanism.” We will discuss in detail “carbanionic mechanism” here. It is termed as carbanionic since the mechanism suggests the production of a carbanion as an intermediate.
The Clemmensen reduction is particularly effective when the starting carbonyl compound is an aryl-alkyl ketone, which could be initially formed via Friedel-Crafts acylation. It is also important to note that the starting aldehyde or ketone must resistant to acidic environment. If so, then the reduction must be done in basic medium, particulary through Wolff-Kishner reaction. In addition, Clemmensen reduction will not reduce carboxylic acid group (-COOH) if present together with the ketone group.
Hence, if you want to reduce an aldehyde or a ketone to an alkane, then you have two options: (1) with acidic environment, Clemmensen reduction is recommended; and (2) in basic medium, Wolff-Kishner reaction is preferred.
Carbanionic mechanism of Clemmensen reduction:
Further examples are shown below: