Oxidation of Aldehydes and Ketones to Carboxylic Acids
OXIDATION OF ALDEHYDES AND KETONES TO CARBOXYLIC ACIDS
Before we discuss how a carboxylic acid is obtained from aldehydes and ketones, let’s revisit first the structural distinction between an aldehyde and a ketone.

Clearly, aldehydes have a hydrogen (H) atom attached to the carbonyl (C=O) group while ketones contain alkyl and/or aryl groups bonded on both sides of the C=O. The presence of hydrogen on aldehydes make them susceptible to oxidation or in another view, they are strong reducing agent. The –CHO proton is being abstracted during the oxidation process. On the other hand, the absence of an H atom next to C=O in ketones make them very difficult to oxidize. You will find the details of oxidation of aldehydes and ketones further on this article.
At milder conditions:
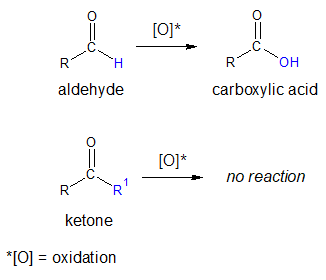
It is also important to mention the oxidizing agents that can be used in the oxidation of aldehydes and ketones. Aldehydes only need milder reagents in order for them to be oxidized while ketones require strong ones.
Table 1. Commonly used aqueous oxidants (oxidizing agents)
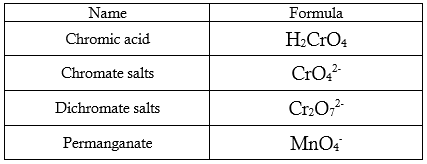
All of the reagents listed in Table 1 can oxidize aldehydes to carboxylic acids, but chromium-containing reagents are the common choice. Permanganate is frequently used in the oxidation of ketones. Although ketones are inert to most oxidants, they can undergo slow cleavage reaction when treated with hot alkaline KMnO4.
Oxidation of Aldehydes to Carboxylic acids
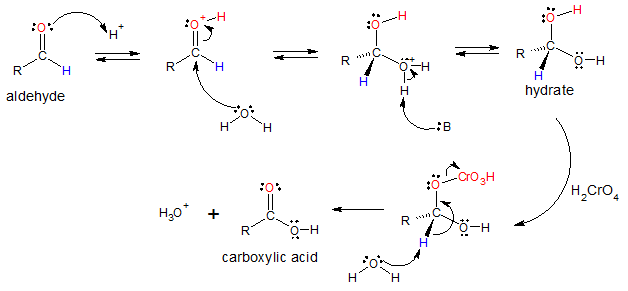
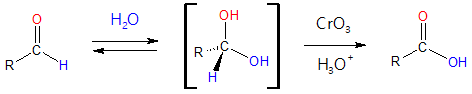
Oxidation of aldehydes occur through 1,1-diol intermediates or also called hydrates, which are formed through a reversible nucleophilic addition of H2O to the carbon of the C=O group. The hydrate produced then reacts in the same manner as primary or secondary alcohols do, they are oxidized to a carbonyl compound.
General reaction:
Oxidation of Ketones to Carboxylic acids
For ketones, the C-C bond next to the C=O is broken, and dicarboxylic acids are produced. The reaction is more ideal for symmetrical ketones because mixture of products is encountered for unsymmetrical ketones.
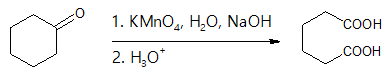
Since generally ketones are difficult to oxidize to carboxylic acids, only the mechanism of aldehydes is illustrated in this discussion.
Mechanism of acid-catalyzed oxidation of aldehydes: