Acetal Hydrolysis
Acetal Hydrolysis
Acetals can be hydrolysed only by using an acid catalyst just as acetal formation requires acid catalysis. With aqueous acid, the hydrolysis of acyclic acetals is very easy. For the given acetal below, it will be hydrolyzed to form back the ketone and alcohol.
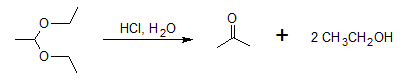
Acid-catalyzed hydrolysis mechanism
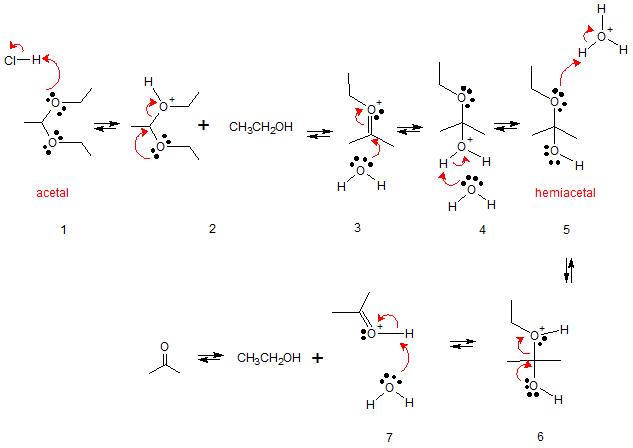
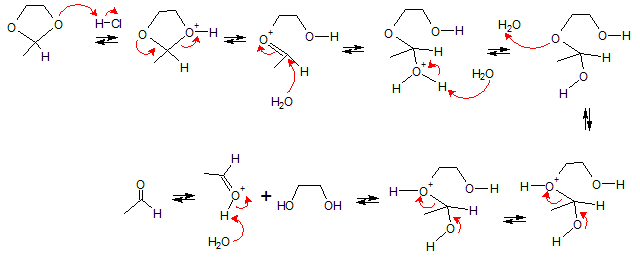
The reaction goes through the following stages. (1) protonation to make a better leaving group (2) lone pair in oxygen allows alcohol leaving group to leave forming oxonium ion (3) water acting as a nucleophile attacks C=O group (4) water then abstracts proton to form hemiacetal (5) protonation again to make a better leaving group (6) lone pair in oxygen allows alcohol leaving group to leave forming oxonium ion
(7) abstraction of proton by base. The end product of the reaction yields the original ketone (or aldehyde) and the excess alcohol.
Hydrolysis of cyclic acetals will yield aldehyde (ketone) and diols. The acid catalyzed reaction mechanism is shown below.
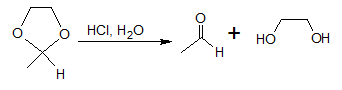
Mechanism for Acid-catalyzed hydrolysis of cyclic acetal