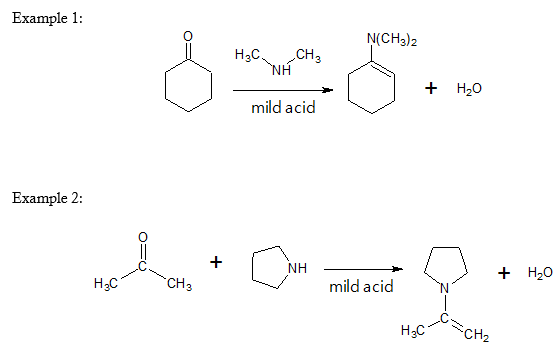
Formation of Enamine
FORMATION OF ENAMINE
You may have already encountered imine formation in the previous article. As a review, imines are formed when primary amines undergo nucleophilic addition with aldehydes and ketones. Now how about enamines? By looking at their names you might think that imines and enamines are chemically related and you’re absolutely right. Both of them are nitrogen-containing organic compounds but structurally speaking, each has distinct features. Shown below are the general structures of imine and enamine.
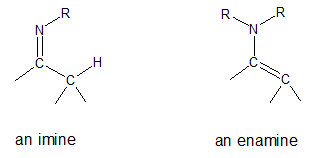
We will focus our attention in this article on enamines. Enamines have a nitrogen atom bonded to a carbon-carbon double bond (alkene + amine = enamine). We can look at enamines as nitrogen analogs of enols (alkene + alcohol).
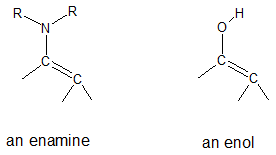
Now that we know the structural features of enamines, our next concern would be how are enamines formed? When a secondary (2o) amine reacts with an aldehyde or ketone, an enamine is formed.
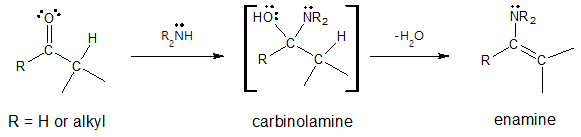
The mechanism for enamine formation is identical to the mechanism with that of imine except for the last step where there is now a carbon-carbon π bond formation involved. When you look at the mechanism for enamine, you can see that it is also a typical example of nucleophilic addition reactions in which small molecule like water is eliminated from the initially formed tetrahedral intermediate and a new C=Nu (Nu as the nucleophile) bond is formed.
Mechanism of Enamine Formation:
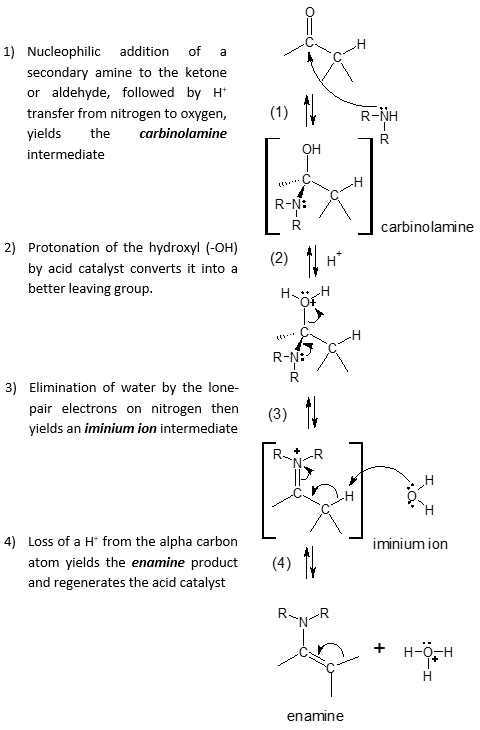
Specific examples of enamine formation are shown below: