General Characteristics of Aldehydes and Ketones
GENERAL CHARACTERISTICS OF ALDEHYDES AND KETONES
Aldehydes and ketones are simple compounds having the carbon-oxygen double bond (-C=O) referred to as carbonyl group. Carbonyl compounds are widespread ranging from carboxylic acids, esters, amides and other carboxylic acid derivatives. Aldehydes and ketones are considered the simplest carbonyl compound in the sense that no other reactive groups such as –OH or –X (halogen) are attached to the carbonyl carbon. At this point, the physical properties and structural features of aldehydes and ketones will be discussed. Understanding these basic concepts will help a lot when you study the reactions of aldehydes and ketones.
Distinguishing between an Aldehyde and a Ketone
An aldehyde differs from a ketone in terms of structure by having a hydrogen atom bonded to the carbonyl carbon. In a ketone, the carbonyl carbon is bonded to two other carbon atoms belonging to alkyl groups of varying complexity. To appreciate this more, look at the examples below.
Table 1. Examples of aldehydes and ketones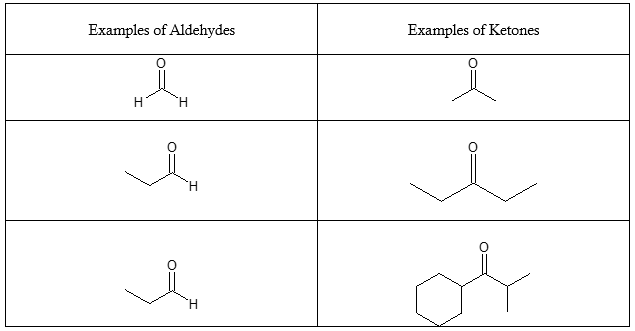
Polarity of Aldehydes and Ketones
The presence of –C=O bond gives aldehydes and ketone a considerable polarity. The oxygen atom is far more electronegative than carbon, allowing the O atom to pull electron density in a –C=O towards itself. This makes the carbon-oxygen double bond highly polar as can be seen in the next figure.

Figure 1. Polarity of carbon-oxygen double bond
As can be seen in the figure, the pull of electron density towards O makes the carbonyl C an electron poor site which is susceptible to nucleophilic attack. This accounts for the fact that aldehydes and ketones nucleophilic addition reactions which will be discussed in other articles.
Table 2. Comparison of physical properties of aldehydes and ketones with their corresponding hydrocarbons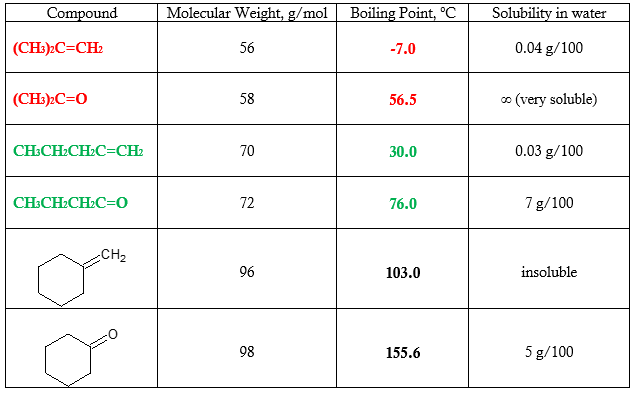
The table above shows a comparison of common properties such as boiling point and solubility in water between aldehyde and ketone compounds and their hydrocarbon analogs. There is an appreciable difference in the boiling point and solubility when you go from hydrocarbon compound to carbonyl counterpart. This can be attributed by the increase in strength of the intermolecular force of attraction from van der Waals forces to dipole-dipole interaction.
