Hydrate Formation (Gem-diols) - Hydration of Aldehydes and Ketones
Hydration of Aldehydes and Ketones
It has been shown that in aqueous solution, there is an equilibrium that exists between an aldehyde or a ketone with its hydrate also known as a geminal diol. The reaction involves nucleophilic addition mechanism and it could be an acid-catalyzed hydration wherein water (H2O) acts the nucleophile or a base-catalyzed hydration with hydroxide ion (OH-) as the electron-rich species.
Before we look at each of the mechanism just mentioned, let’s compare the extent of hydration between aldehydes and ketones.
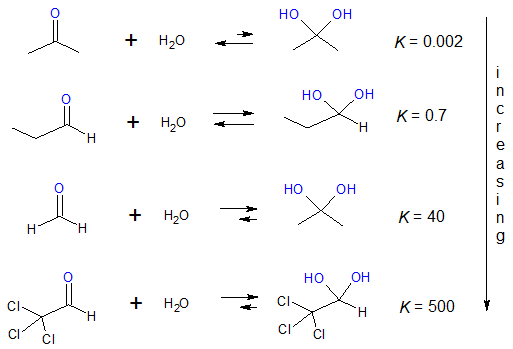
As a review, the equilibrium constant K of a reaction tells us about the extent of the reaction. Since equilibrium reaction is involved, it is expected that the reactants and products coexist when state of equilibrium is reached. The value of K allows one to assess which side of the reaction predominates at equilibrium. Notice an increase in the value of K as we go from hydration of ketone to chlorinated aldehydes.
It can be said that the hydrate form of most ketones is not favoured and mostly the keto form is present at equilibrium. Aldehydes, on the other hand, are more likely to form stable hydrates. There is a stabilizing effect to the electrophilic carbonyl group with the presence of two electron-donating alkyl groups in ketones, while in aldehydes there is only one stabilizing alkyl group. In this sense, aldehydes are more electrophilic and less stable than ketones. In fact, formaldehyde is more easily hydrated than any other aldehydes.
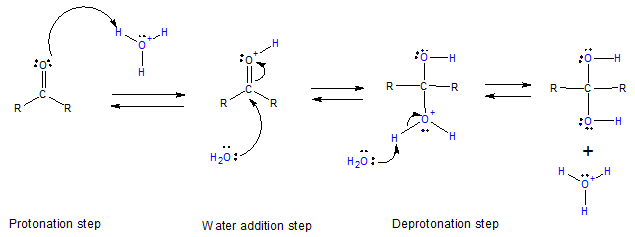
Acid-catalyzed Hydration
The acid-catalyzed hydration is a typical acid-catalyzed addition to the C=O group. Protonation followed by addition of water, resulting to a protonated product. Deprotonation yields the hydrate accompanied by regeneration of the acid catalyst.
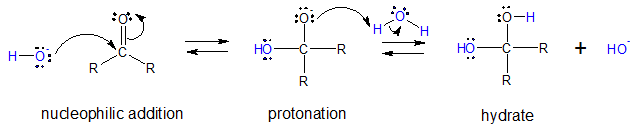
The base-catalyzed addition to the C=O group of a ketone or aldehyde is a more direct way of forming geminal diols since it only consist of nucleophile addition and then protonation.
Another consideration wherein the formation of hydrates is favoured is when electron-withdrawing groups are attached to the alkyl group bonded to the C=O group. For instance, in the last reaction shown previously involving Cl-containing alkyl group of the aldehyde, notice the drastic shift of the equilibrium constant to 500 which implies that the hydrate form is more stabilized and thus, predominates in the reaction. The presence of an electronegative atom like Cl destabilizes the partially positive carbon of the C=O group, making it more electron deficient and more vulnerable to nucleophilic addition.
