Sn2 Reaction and Characteristics
Introduction to Nucleophilic Substitution Reaction
Nucleophilic substitution reactions are a class of reactions where one nucleophile displaces another.

The molecule on which the substitution takes place is called the substrate, while the group from the substrate which gets expelled by the incoming nucleophile is called the leaving group. These reactions are governed by several factors which dictate the rate and mechanism of these reactions. The very first thing to learn about these reactions is that these reactions occur due to the polarity of the bond between the carbon atom and the leaving group.
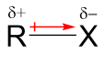
Due to the partial positive charge on the carbon atom, the nucleophile attacks the carbon atom and pushes its electron pair to break the bond between the carbon atom and the leaving group.
The Bimolecular Nucleophilic Substitution Reaction (SN2)
The SN2 reaction is a single-step reaction with a concerted mechanism: Bond cleavage and bond formation occur simultaneously.

The reaction takes place via a pentavalent carbon transition state which includes both the substrate, the nucleophile, and the leaving group.
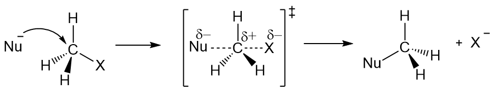
Since the transition state includes the alkyl halides and the nucleophile, the reaction is bimolecular (or second-order). The rate of the reaction depends on both the substrate and the alkyl halide.
Rate = k[RX][Nu-]
Stereochemistry of the Reaction
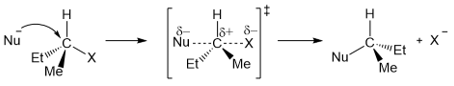
The SN2 reaction results in the inversion of configuration at the carbon being attacked by the nucleophile. The nucleophile attacks from the rear side of the leaving group and causes a flip in the configuration of the carbon atom. The process of analogous to an umbrella being flipped inside out on a windy day.
Factors Affecting the Rate of SN2 Reaction
Since SN2 reaction is a bimolecular reaction, the rate of reaction is dependent upon both the nucleophile and the substrate.
Strength of the Nucleophile
Stronger nucleophiles react faster in SN2 reactions. The strength of a nucleophile depends on various factors such as electronegativity, charge, size, steric hindrance, and resonance effect. As a first rule, nucleophilicity decreases with increasing electronegativity. For example, nitrogen has a lower electronegativity than oxygen. Therefore, nitrogen is more willing to give up electrons than oxygen. This means that between H2O and NH3, NH3 will be a stronger nucleophile. As a periodic trend, nucleophilicity decreases from left to right in a period.
Nucleophilic strength increases with increasing atomic size. Larger anions are more polarizable and therefore more nucleophilic. As a rule, nucleophilic strength increases down a group.
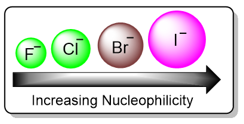
Effect of the Leaving Group
If a leaving group is bonded tightly to the carbon atom being attacked by the nucleophile, it will decrease the rate of an SN2 reaction. Leaving groups with weaker bonds are good leaving groups while leaving groups with stronger bonds are bad leaving groups. Take the example of halogens, fluorine due to its high electronegativity and small size forms strong bonds with carbon while iodine forms the weakest bonds. As a periodic trend, leaving group ability increases down a group.
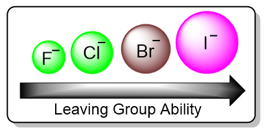
Steric Hindrance as a Factor for SN2 Reaction
Steric hindrance is important for both the nucleophile and the substrate. Since the nucleophile has to approach the carbon atom from the rear side of the leaving group, its size is a contributing factor to the rate of the reaction. Larger nucleophiles have difficulty approaching the carbon atom, therefore larger nucleophiles react slower than smaller nucleophiles.
For substrate, the substitution pattern at the carbon atom is a major contributing factor. Primary substrates react the fastest in SN2 reactions while tertiary substrates react the slowest in SN2 reactions. Below is an example containing three reactions. In each of the reactions, the same nucleophile replaces the same leaving group. The only deciding factor in these three reactions is the steric hindrance at the carbon atom where the substitution is taking place.
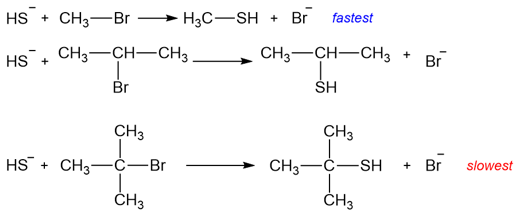
SN2 Reaction and the Effect of Solvent
A solvent plays an important role in the kinetics of any chemical reaction. Not only the solvent can alter the rate of a reaction, but sometimes it can even change the outcome of a reaction. To understand the effect of a solvent on an SN2 reaction, we have to first recall how the nucleophilic strength alters the rate of an SN2 reaction.
We know that the rate of an SN2 reaction depends upon the strength of the nucleophile. The stronger the nucleophile, the faster the rate. The solvent alters the rate of an SN2 reaction by altering the strength of the nucleophile.
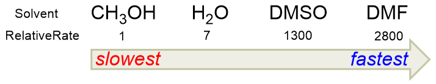
The best solvents for SN2 reactions are polar aprotic solvents while polar protic solvents are the worst solvents for these reactions. Polar protic solvents such as water can solvate the nucleophile and decrease its ground energy. As a result, its nucleophilicity (nucleophilic strength) is decreased.
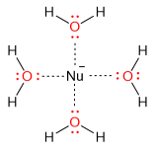
Polar aprotic solvents (such as DMF or DMSO) are the ones that have dipoles, but they do not have protons (-OH or -NH). As a result, these solvents can only solvate the cations while leaving the anions “free” or “naked”. The ground state energy of the anions is raised because they are not hydrated and their nucleophilicity is increased. Below is an example of SN2 substitution of bromide by the azide anion.

The rate of reaction is the slowest in polar protic solvents (water and methanol) while it is the fastest in polar aprotic solvents (DMF and DMSO).