Rearrangement Considerations for Sn1
Rearrangement Considerations for Sn1
Carbocations often undergo structural changes to form more stable carbocations in a process called rearrangement. Rearrangements involve the breaking of either a C−H bond or a C−C bond. The energy required to break the C−H or the C−C bond is compensated in terms of the carbocation stability at least partially if not fully.
Carbocation rearrangements do not take place in SN2 reactions due to the one-step concerted mechanism. The bond breaking with the leaving group and bond formation with the nucleophile take place simultaneously and no carbocation is formed. However, SN1 reactions show an aptitude for carbocation rearrangements. It is known that primary alkyl halide substrates do not usually react via the SN1 mechanism. But in the case when the approach to the backside of the carbon that bears the leaving group is too hindered and there is a possibility of a rearrangement, the reaction can take place via the SN1 mechanism.
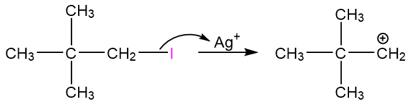
Take an example of the silver-promoted substitution of neopentyl iodide.
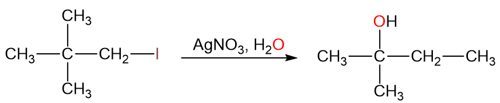
As can be seen in this reaction, the carbon skeleton of the product than that of the reactant. We will come back to the mechanism of this reaction, in a later section.
Hydride Shifts
Many carbocation rearrangements take via the breaking of the C−H bond whereby a hydrogen atom migrates from one carbon atom to another, with a pair of electrons (hence the term “hydride shift”).
An example of such a rearrangement is the reaction of 2-bromo-3-methylbutane with methanol to give two substitution products.
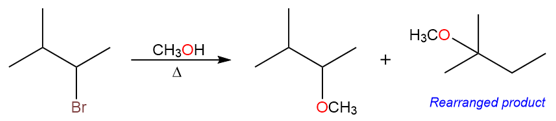
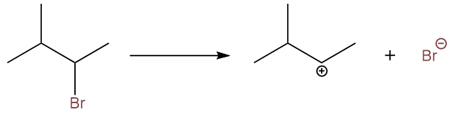
The reaction takes place via an SN1 mechanism. The bromo group leaves first creating a secondary carbocation.
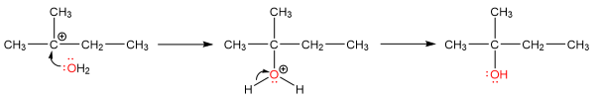
The unrearranged product is formed when the methanol attacks the secondary carbocation.
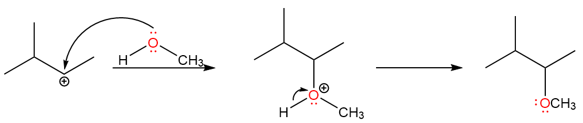
The rearranged product is formed by a hydride shift producing a tertiary carbocation which is subsequently attacked by methanol.
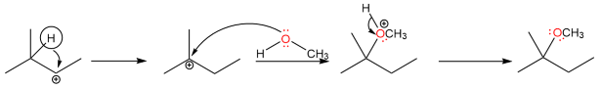
Methyl Shifts
Rearrangement to form a more stable carbocation can also take place via a methyl shift. Let’s get back to the reaction we encountered earlier – the silver-promoted substitution of neopentyl iodide.
Halides have a great affinity for silver because they form stable and insoluble silver halides.
The primary carbocation formed here is too unstable to exist but it has the potential to rearrange into a more stable tertiary carbocation via a methyl shift.
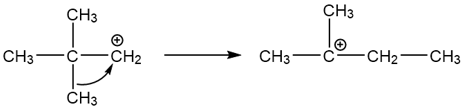
The tertiary carbocation is then attacked by water to form the rearranged alcohol product.
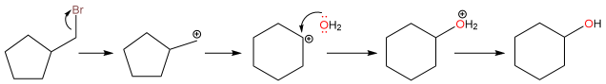
Other Alkyl Shifts
When a C−C bond is broken to form a rearrangement product, a methyl group doesn't need to migrate. Sometimes, a ring expansion can also take place.
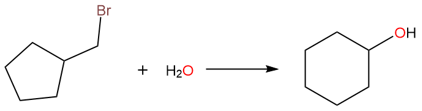
Although there isn’t a great difference in stability between a five-membered and a six-membered ring, the rearrangement seen in this reaction is due to the difference in carbocation stabilities. When the bromide ion leaves, a primary carbocation is formed. The primary carbocation then rearranges to a secondary carbocation through ring expansion. Finally, the attack of water and then the removal of a proton yields the rearranged alcohol product.