Sn1 Reaction and Characteristics
Unimolecular Nucleophilic Substitution Reaction (SN1)
Unimolecular substitution reactions occur on substrates where the back side of the leaving group is not accessible. In general, tertiary alkyl halides react the best in SN1 reactions.
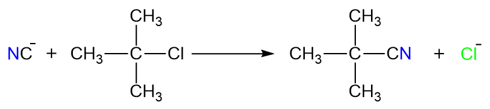
These reactions take place in two steps and proceed via a carbocation intermediate. The first step of the reaction is the formation of a carbocation after the removal of the leaving group (usually a halide ion). The carbocation is then attacked by the nucleophile forming the final substituted product.
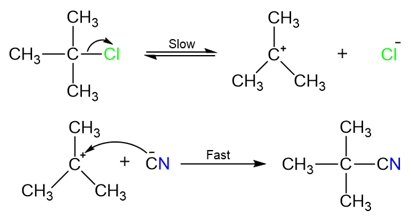
The rate-determining (slow) step of the reaction is the first step where the halide ion is removed and the carbocation is formed.
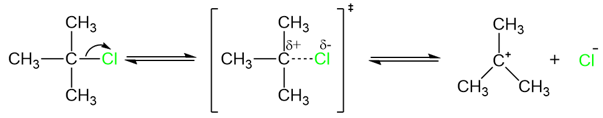
Since the transition state of the slow step only involves the substrate (alkyl halide), the rate of the reaction depends only on the concentration of the alkyl halide. The reaction is a first-order reaction.
Rate = k[RX]
Stereochemistry of the Reaction
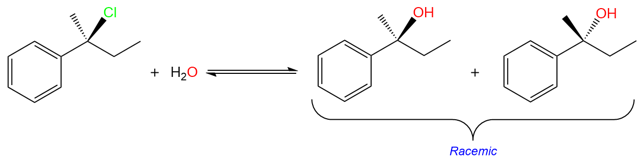
In addition to the mechanism, the SN1 reaction is also different from the SN2 reaction in terms of the product stereochemistry. This reaction results in the racemization of the stereocenter at which the substitution takes place.
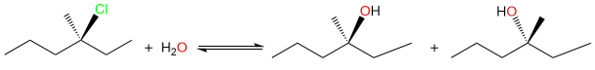
The racemization is due to the formation of the planar carbocation. Unlike the SN2 reaction where the nucleophile only attacks from the back side, the nucleophile in SN1 reaction can attack the carbocation from either side to create two different enantiomers in equal amounts (racemization).
Factors Affecting the Rate of SN1 Reaction
The SN1 reaction takes place in two steps: Generation of the carbocation and the reation of carbocation with the nucleuophile to yield the final product. The first step of the reaction is the rate-determining step and that does not include the nucleophile. So the rate of an SN1 reaction is unaffected by the concentration or the nucleophilicity of the nucleophile.
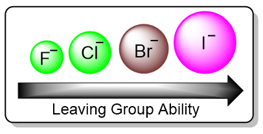
Leaving Group Effect
The transition state for the slow step of an SN1 reaction includes the substrate with the leaving group partially attached to the prospective carbocation. The rate of this step depends upon how fast or slow the bond between the carbon atom and the leaving group breaks. Substates with good leaving groups react fast while substrates with poor leaving groups react slowly. The leaving group ability is dependent upon the electronegativity and the size of the leaving group. Highly electronegative or small atoms are poor leaving groups while atoms with low electronegativty and large size are good leaving groups.
Nature of the Substrate
The nature of the substate itself is a major deciding factor in the SN1 reactivity of a molecule. Substrates that can generate stable carbocations react easily under SN1 reactions. A general trend of carbocation stability is shown below:
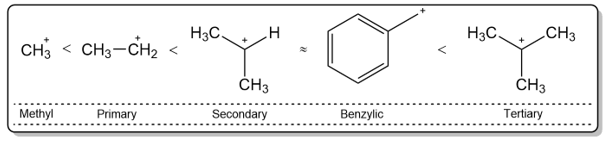
The reason behind the stability of secondary and tertiary carbocations is hyperconjugation. Hyperconjugation is the name given to a stabilizing interaction between a hybridized filled orbital and an unhybridized empty orbital.
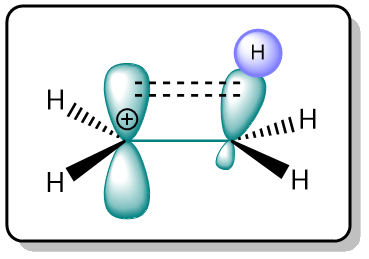
As can be seen from the figure above, the electrons in the C–H σ-bonds stabilize the empty p-orbital of the carbocation. The greater the number of these C–H σ-bonds, the greater the stability. That is why tertiary carbocation are the most stable because they provide the greatest degree of hyperconjugation.
Solvent Effect
The SN1 reactions are similar to the SN2 reactions in a way that their rates are dependent upon the nature of the solvent. However, the reactivity trend is entirely opposite: SN1 reactions proceed the fastest in polar protic solvents. This trend in reactivity is due to the mechanism of the SN1 reaction. Since the mechanism of an SN1 reaction involves the formation of a carbocation, a solvent that can facilitate the bond cleavage between the carbon atom and the solvate works best in these reactions.
Polar protic solvent increase the rate of SN1 reactions because they can solvate both the carbocation and the leaving group. This solvation process results in the weakening of the bond between the carbon atoms and the leaving group and the rate of the reaction is increased. The figure below shows how water, a polar protic solvent, can solvent both a carbocation and a leaving group.
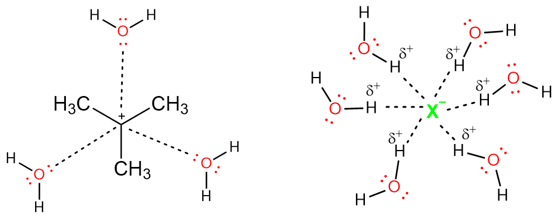
Some Notable Features of the Solvents
Not only a solvent can alter the rate of a substitution reaction, it can even alter the mechanism of the reaction for some substrates. It has been discussed that primary substrates cannot react under SN1 conditions due to the unstable nature of primary carbocations and that tertiary substrates react preferably by the SN1 mechanism. But what about secondary substrates? Secondary carbocations are neither very unstable nor very stable. In most cases, secondary substrates react via both mechanisms. However, the predominant mechanism is decided by the nature of the solvent.
Polar protic solvents promote the SN1 mechanism while polar aprotic solvents promote the SN2 mechanism.
Take an example of this benzylic substate.
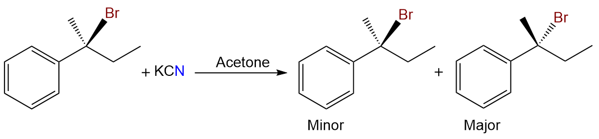
When this reaction is carried out in acetone (an aprotic solvent or medium polarity), the major product is the one where inversion has taken place. Although there is some SN1 mechanism operating because as evident by the product with retained configuration, the major mechanism operating here is SN2. This shows how a polar aprotic solvent has shifted the choice of mechanism for a secondary substate.
Here's an example of how a polar protic solvent (H2O) shifts the mechanism towards SN1 and results in racemization of the product.