Rearrangement Considerations for E1
Carbocation Rearrangements in E1 Reactions
Just like SN1 reactions, E1 reactions are prone to carbocation rearrangements. The carbocation that is formed after the leaving group is removed, can undergo several rearrangements such as hydride shifts, methyl shifts, or ring expansions. Carbocation rearrangements do not take place in E2 reactions due to the concerted reaction mechanism. The transition state in an E2 reaction includes partial charges spread over the whole transition state but no carbocations are formed. As a result, rearrangements are never seen in E2 reactions.
Hydride and Alkyl Shifts
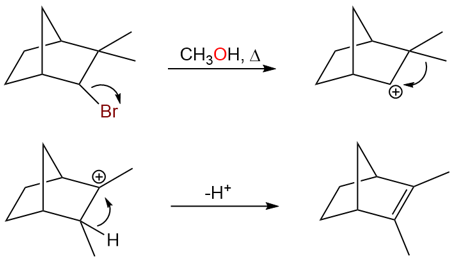
Hydride and alkyl shifts are very common in carbocation rearrangements and they occur when a more substituted carbon atom, adjacent to the carbocation bears a hydrogen atom or an alkyl group. Take an example of the following reaction:
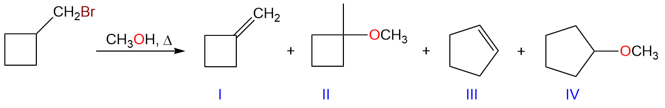
In this reaction, products I and III are elimination products formed due to a hydride shift and a methyl shift, respectively. Products I and II are formed from the same carbocation while products III and IV are formed from the same carbocation.
The mechanism for the carbocation rearrangement is:
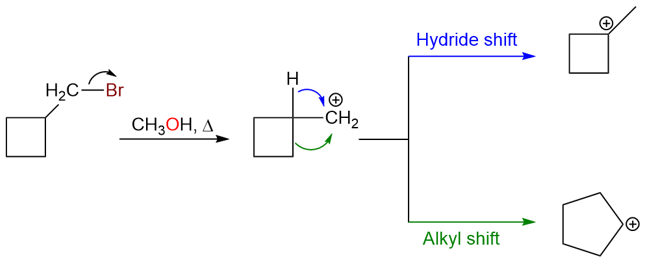
Another example of a methyl shift is the following reaction:
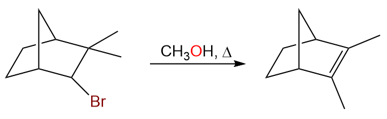
The mechanism for the rearrangement is:
Rearrangement of Tertiary Carbocations
A tertiary carbocation can rearrange into another tertiary (or in some cases secondary) carbocation to relieve some ring strain. This type of rearrangement often happens in bicyclic systems.
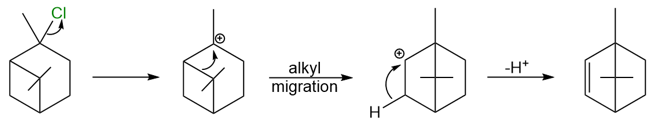
The reaction above shows a tertiary carbocation rearranging to a secondary carbocation. Although the secondary carbocation is less stable than the tertiary carbocation, the relief from the ring strain due to the expansion of the four-membered ring into a five-membered ring imparts stability to the system.
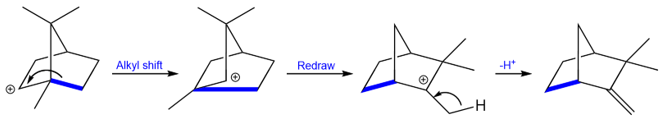
Rearrangement of Isoborneol to Camphene
We will close this section with a famous rearrangement reaction that shows how some rearrangements look very complex but are fairly simple to understand.
The reaction starts with the protonation of the alcohol to form water which is an excellent leaving group.
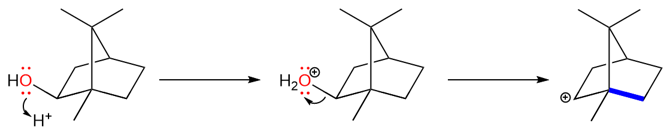
The carbocation that is formed is a secondary carbocation but it cannot convert into a tertiary carbocation by a methyl shift because that would generate a carbocation at a bridgehead. Carbocations at bridgeheads can never be planar and therefore are too unstable to exist.
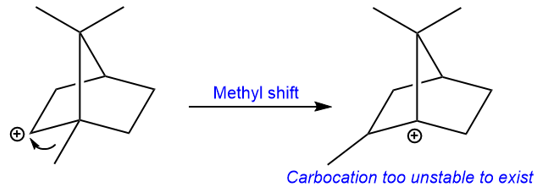
Instead of the methyl group migration, the bond highlighted in blue migrates to form another tertiary carbocation. The loss of a proton from the methyl group next to the carbocation results in an exocyclic double bond.