Alkyne Formation via Elimination
Alkyne Formation via Elimination
We have already seen in previous sections that elimination reactions can result in the formation of alkenes. Alkynes can be prepared by elimination reactions, just like alkenes. The easiest method for the preparation of alkynes by elimination is the double dehydrohalogenation of a vicinal or geminal dihalide.
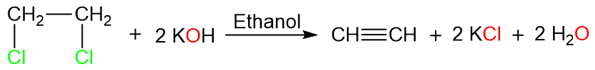
The reaction above is a very simple reaction that converts 1,2-dichloroethane into ethyne. However, the yield for this reaction is generally low. The reason for the low yield lies in the pKa value of the protons being removed by the base. The reaction takes place in two steps: In the first step, the removal of a proton by the base yield vinyl chloride.
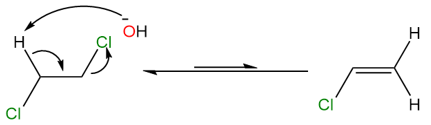
The removal of another proton from vinyl chloride, yields the final alkyne product.
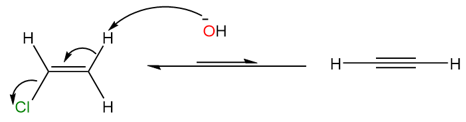
As seen from the two reaction steps above, the protons being removed by the base are alkyl and vinylic which have pKa values of ~51 and ~44, respectively. However, the pKa value of hydroxide bases is generally around 15. To overcome this energy barrier, heat must be provided to the reaction and that is why the yields are generally very low when hydroxide or similar bases are used. To increase the yield and reaction rate, very strong bases such as sodamide (NaNH2) or lithium alkylamides (Li+R2N−) are generally used.
Effect of Stereochemistry on Reaction Rate
Sometimes the formation of the alkyne product from two isomers takes place at different reaction rates. The reaction of (1R,2R)-1,2-dibromo-1,2-diphenylethane with KOH takes place at a faster rate than the reaction of (1R,2S)-1,2-dibromo-1,2-diphenylethane.
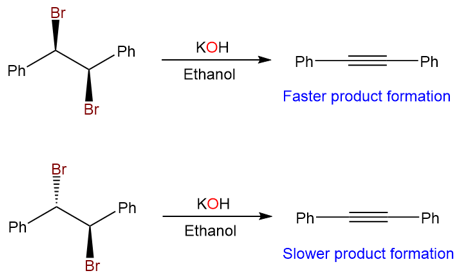
The reason lies in the intermediate that is formed as a result of the first elimination. Let’s have a look at the mechanism for the elimination reaction of (1R,2R)-1,2-dibromo-1,2-diphenylethane.
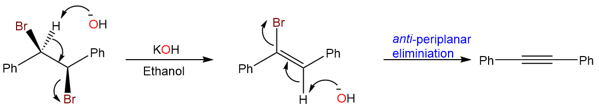
The removal of the vinylic proton in this reaction takes place via an anti-periplanar transition state. Due to low steric hinderance in the anti-periplanar transition state, it is more stable and leads to a faster reaction rate.
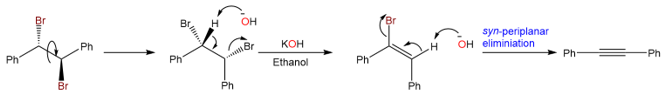
The reaction of (1R,2S)-1,2-dibromo-1,2-diphenylethane takes place at a much slower pace because the first elimination requires the rotation of the sigma bond, and the transition state leading to the cis alkene intermediate is sterically hindered. Furthermore, the second elimination takes place via a syn-periplanar elimination. The second transition state is also crowded and results in an overall decrease in the reaction rate.
