E1 Reaction and Characteristics
E1 Reaction and Characteristics
Unimolecular Elimination Reaction (E1)
Unimolecular elimination reactions are in two ways similar to the unimolecular substitution reactions (SN1): 1) They take place in two steps, and 2) They proceed through a carbocation intermediate.
Take an example of the elimination reaction of isobutene from tert-butyl chloride.
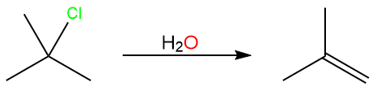
The reaction starts with the removal of the leaving group and a carbocation is formed:
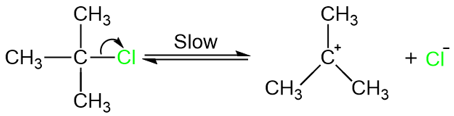
E1 reactions often occur in competition with SN1 reactions. Water in this reaction can act as either a nucleophile or a base. We do not have much control over how the reaction goes except for one factor: The temperature. Since eliminations are entropy favored and take place at higher temperatures, we can assume that this reaction is being carried out at a higher temperature and water is acting more as a base and less as a nucleophile.
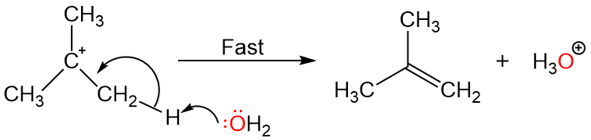
The slow (rate-determining) step of this reaction is the first step: The formation of the carbocation.
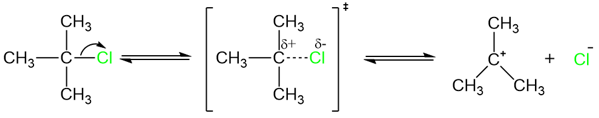
The reaction is first order because the transition state for the rate-determining step is unimolecular.
Rate = k[RX]
Stereochemistry Of E1 Reaction
E1 reactions are stereoselective and regioselective. These reactions usually result in a more substituted (Zaitsev) and trans alkene.
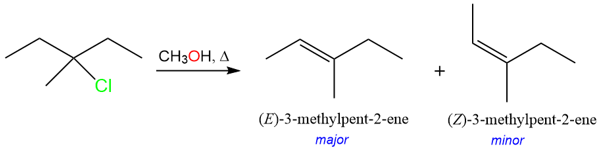
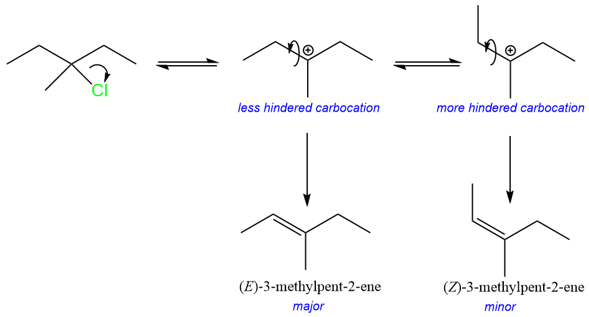
The observed stereoselectivity is because the carbocation can adopt a more favorable, lower energy conformation before losing a proton to yield the alkene product.
Factors Affecting Rate of E1 Reaction
E1 reaction take place in two steps: 1) Formation of the carbocation, 2) removal of proton to form the alkene. The rate-determining (slow) step does not involve the base, so the rate of an E1 reaction is independent of the concentration of the base. Although the nature of the base is somewhat important because strong bases tend to shift the reaction mechanism towards E2.
Nature of the Substrate (Carbocation Stability)
The E1 reaction mechanism involves the formation of a carbocation. Substrates that form unstable carbocations, do not react under E1 conditions (unless they can rearrange to form stable carbocations). E1 reactions work best for tertiary substrates while primary substrates fail to react under E1 reaction conditions.
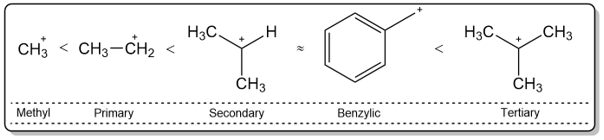
The reason behind the stability of tertiary carbocations is hyperconjugation (As discussed in the SN1 reactions section).
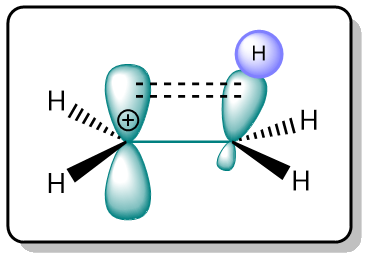
The higher the number of filled C–H σ-bonds adjacent to an empty p-orbital, the higher the stability.
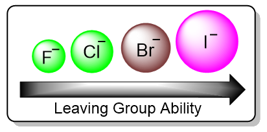
Nature of the Leaving Group
In the E1 reaction mechanism, the rate-determining step involves breaking the bond between the carbon and the leaving group. A leaving group with a strong bond will decrease the rate of the reaction. In the case of the halogens, the C–X bond strength decreases down the group.
This is due to two factors: 1) The atomic radii of halogens increase down the group. Longer bonds are weaker than shorter bonds. 2) The stability of the anion that is formed after the C–X bond has broken is another factor. The larger the atom, the more polarizable it is and the more stable it is. Stable ions tend to exist more easily in solutions.
In the case of leaving groups other than halogens, resonance also plays an important role. Resonance stabilized leaving groups are always good leaving groups.
For example, the tosylate leaving group is a very good leaving group due to its high stability.
Nature of solvent
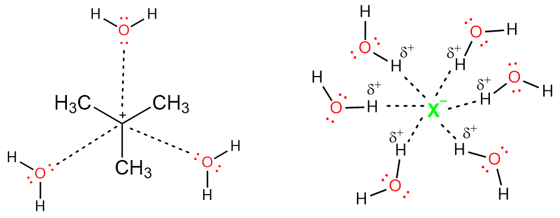
Polar protic solvents are preferred for E1 reactions. Since the reaction mechanism involves the formation of carbocations, a solvent that can stabilize the carbocation increase the rate of the reaction.
Take the example of water, it can solvate both a carbocation and the anion formed as a result of bond cleavage in the first step of the E1 reaction. Water solvates both ions and decreases their ground state energy. This stabilizing interaction weakens the bond between the carbon and the leaving group and increases the rate of an E1 Reaction.
Effect of Temperature
E1 reactions take place at a faster pace at higher temperatures for two reasons: 1) Temperature increases entropy and elimination reactions are entropy-favored. 2) The energy transferred to the reaction medium at a higher temperature is utilized to break the bond between carbon and the leaving group.
