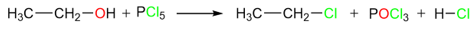Synthesis of Alkyl Halides
Alkyl Halides
Alkyl halides are an important class of organic compounds that serve as precursors to many other organic compound classes such as alcohols, thiols, nitriles, esters, carboxylic acids, etc.
Alkyl halides can be considered as derivatives of alkanes where a halogen atom has replaced one or more of the hydrogen atoms.
Preparation of Alkyl Halides
Alkanes are considered unreactive compounds, but under the conditions of radical halogenation, alkanes can be made to react with halogens to form alkyl halides. Radical halogenation is normally initiated by light and is usually an exothermic reaction.
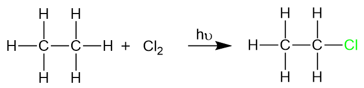
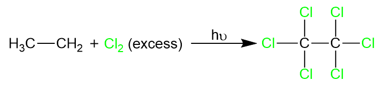
Below is an example of chlorination of ethane to form ethyl chloride:
However radical halogenation isn’t an effective method for alkyl halide synthesis because of the variable reactivities of different halogens. For example, fluorination and chlorination reactions cannot be stopped at monohalogenation due to the highly reactive nature of these two halogens. As a result, we usually end up with a mixture of halogenation products.
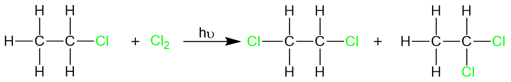
In cases where an excess of halogen is present, the reaction does not stop until all the hydrogen atoms are replaced by halogen atoms.
In the case of iodine, the reaction proceeds so slow that the formation of the product is barely noticeable.
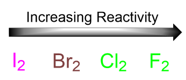
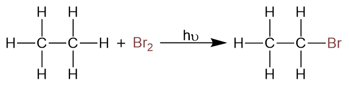
Thus, radical bromination is the only radical halogenation reaction that is controllable and predictable.
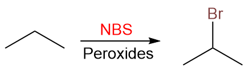
Bromination can be made even more controllable if NBS or similar reagents are used. NBS slowly breaks down in the reaction medium to release bromine radicals that react with the alkane to produce alkyl bromides.
Alkyl Halide Synthesis using Alkenes
A good method for the formation of alkyl halides is the addition of hydrogen halides to alkenes.
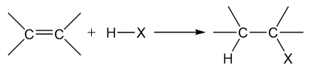
Not only this reaction provides good yields of alkyl halides, but it is also regioselective. Regioselectivity of alkene addition is governed by Markovnikov’s rule. Addition to asymmetric alkenes results in the formation of a more substituted alkyl halide:
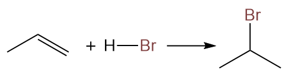
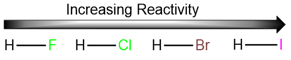
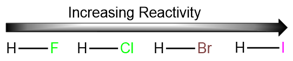
Reactivities of hydrogen halides in radical halogenation reactions
We won’t be discussing the mechanism of hydrogen halide addition in detail here, but it is a good idea to learn the general trend of hydrogen halide reactivity towards alkenes. Since the first step of this reaction is the protonation of the alkene, the rate of formation of the carbocation is dependent upon the strength of the hydrogen-halogen bond. The weaker the bond, the stronger the acid and the faster the addition reaction.
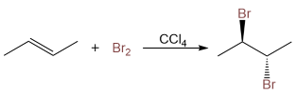
Dihalide Preparation Using Halogens and Alkenes
The addition of halogens to alkenes gives vicinal dihalides. Vicinal dihalides are compounds that have halogen atoms on two adjacent carbon atoms.
Addition of Hydrogen Halides to Alkynes
Alkenes can add two moles of hydrogen halides to produce geminal dihalides. Geminal dihalides are compounds that have two halogen atoms on the same carbon atom.
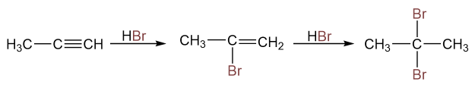
Like alkenes, the addition of hydrogen halide to an alkyne (if asymmetric) follows Markovnikov’s rule.
Reaction of HX with Alcohols
Alcohols react with hydrogen halides to produce alkyl halides.
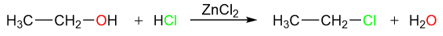
However, this reaction is not equally applicable to the synthesis of all types of alkyl halides. For example, alkyl fluorides cannot be prepared using this method. Like the addition of hydrogen halides to alkenes, this reaction also starts with a protonation step.
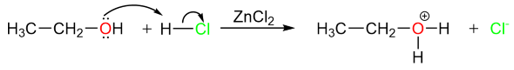
The rate of this step is dependent upon the acidic strength of the hydrogen halide. So the trend of reactivity is the same as alkenes.
However, due to the unstable nature of primary carbocations, the loss of a water molecule from the carbocation is difficult. As a result, primary alkyl halides are difficult to prepare using this reaction. Tertiary alcohols react the fastest with hydrogen halides.
Reaction of Alcohols with SOCl2
Thionyl chloride is an excellent reagent to convert alcohols into alkyl halides under mild conditions. This reaction does not require a high temperature and highly acidic medium (as in the reaction with hydrogen halides) to convert alcohols to alkyl halides. Unlike the reaction with hydrogen halides, primary alcohols react the fastest with SOCl2 followed by secondary alcohols. Tertiary alcohols react the slowest.

Reaction of Alcohols with PCl3/PBr3
Phosphorous trichloride (PCl3) and phosphorous tribromide (PBr3) are another couple of excellent reagents to prepare alkyl halides from alcohols. Stoichiometrically speaking, this reaction is chemical economical: One mole of phosphorous trihalide can convert three moles of alcohol to an alkyl halide.

Reaction of Alcohols with PCl5
Another great reagent for the conversion of alcohols to alkyl halides is phosphorous pentachloride. An advantage of this reaction is that alcohols can be converted into alkyl halides under neutral conditions and at low temperatures. A disadvantage would be the violent nature of this reaction at higher temperatures.