Zaitsev Vs Hoffman Elimination
Zaitsev’s Rule and Elimination Reactions
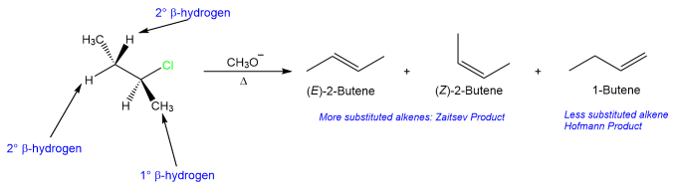
We have seen in previous sections that elimination reactions exhibit regioselectivity. In many cases, an elimination reaction (whether E1 or E2) produces a more substituted alkene. Zaitsev’s rule states that the alkene that is formed in the highest amount in an elimination reaction is the one that comes from the removal of hydrogen from the β-carbon that bears the least number of hydrogen atoms. This means that the removal of the β-hydrogen is preferred from the carbon that has a higher degree of substitution. This can be seen from the reaction below. The major product (a mixture of (E)-2-butene and (Z)-2-butene) is the one that comes from the removal of a 2° β-hydrogen.
Alkene Stability
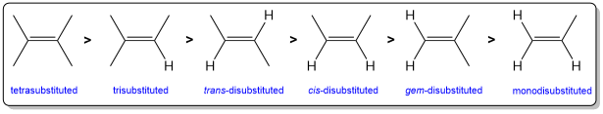
Zaitsev’s rule can be stated alternately: “The major product of an elimination reaction is the more substituted alkene”. The stability trend of substituted alkenes is:
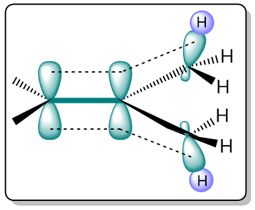
The reason behind the increased stability of higher-substituted alkenes is hyperconjugation. Higher substituted alkenes have a greater number of C−H σ−bonds that can stabilize an alkene through orbital interactions.
E2 Reactions using Small Bases Prefer the Zaitsev Product
Although β-hydrogens at higher substituted carbons are not very easily accessible, small bases tend to preferably remove them to form Zaitsev products.
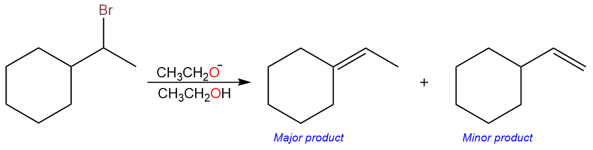
In this reaction, the major product is a trisubstituted alkene while the minor product is a monosubstituted alkene. The formation of both products is shown below:
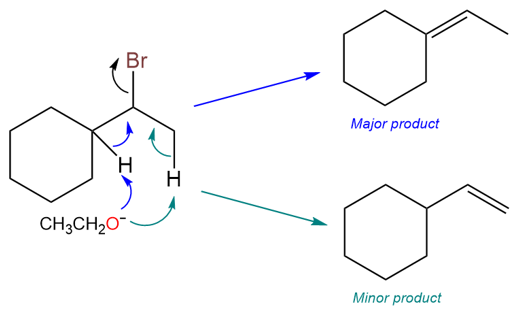
E1 Reactions Always Prefer the Zaitsev Product
E1 reaction take place via a carbocation intermediate and therefore, the carbocation has plenty of time to adopt a low energy conformation that leads to the formation of a trans and more substituted product.
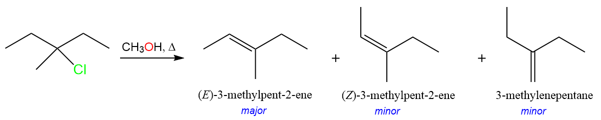
Bulky Bases Prefer the Hofmann Product
Strong bulky bases such as tert-butoxide are too large to approach the take the proton at a higher degree β-carbon. These bases preferentially remove the outermost proton to avoid crowding in the transition state and therefore result in the formation of the least substituted product.
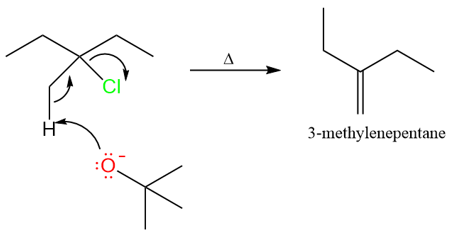
Regioselectivity Due to Anti-Periplanarity
Until now we have seen some very clear examples of eliminations. Small bases give Zaitsev products and bulky bases give Hofmann products. However, there are cases when small bases give exclusively Hofmann products.
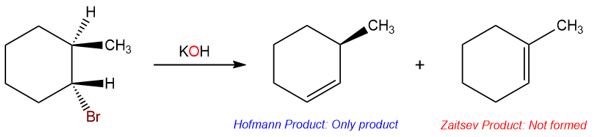
The reason for the Hofmann product in this reaction is the anti-periplanar requirement for the E2 reaction. Under E2 conditions, the proton that is removed by the base must be anti-periplanar to the halogen leaving group.
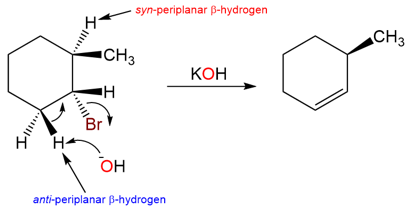
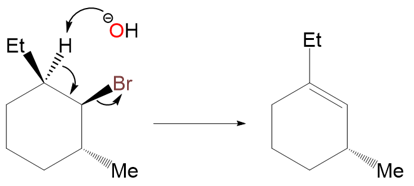
Take another example of a similarly substituted cyclohexane:
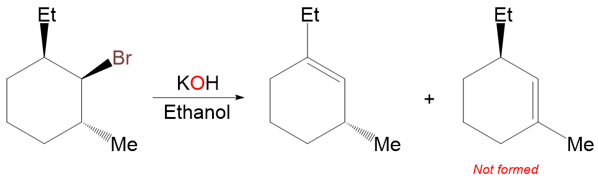
Both of the alkene products shown above are trisubstituted, but the second product cannot form due to the stereospecific nature of the E2 reaction. There is only one anti-periplanar β‑hydrogen present. It’s on the carbon atom that bears the ethyl group.