Intramolecular Substitution Reactions
Introduction to Intramolecular Reactions
Intramolecular reactions are a class of reactions where the two reacting moieties/species are within the same molecule. In other words, a single molecule reacts with itself. In the context of substitution reactions, the nucleophile and the leaving group are within a single molecule. Let’s start by taking examples from some common reactions.
Epoxide synthesis using halohydrins
Alkenes are unsaturated molecules which can add various reagents such as hydrogen, water, halogens, hydrogen halides, etc. Addition of halogens to alkenes in aqueous media result in the formation of halohydrins.
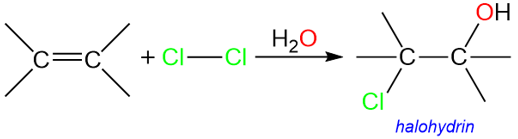
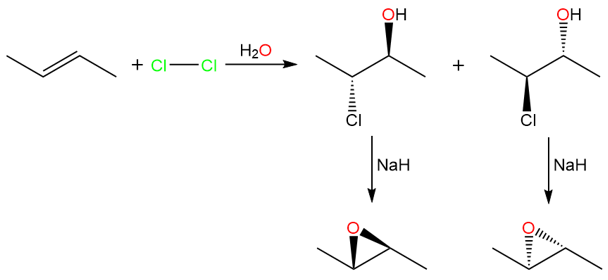
A halohydrin is a classic example of a molecule that has both a leaving group (−Cl) and a nucleophile (−OH) in a single molecule. Halohydrins, under strongly basic conditions, yield epoxides (3-membered cyclic ethers).
Formation of Five and Six-Membered Rings
Due to large ring strain, three and four membered rings are a bit difficult to prepare using substitution reactions. On the other hand, it is easier to prepare five and six membered rings using substitution reactions. Five-membered rings have low ring strain while six-membered rings are generally considered strain-free. A word of caution: the substituents on the rings can have a major impact on the ring strain.
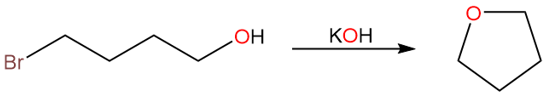
The example given above is for an SN2 reaction where the alkoxide ion formed by deprotonation of the hydroxyl group attacks on the carbon bearing the bromine atom to form the final cyclic ether.
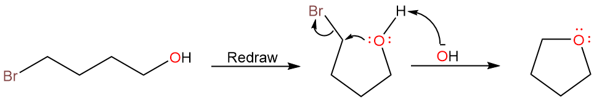
Since this is an SN2 reaction, the transition state includes both the substrate and leaving group.
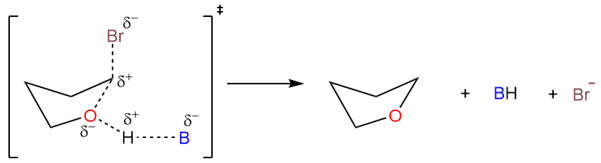
SN2 Vs. SN1 in Intramolecular Substitutions
The factors that dictate the reaction mechanism in intramolecular substitution reactions are the same as they are for “usual” substitution reactions. Tertiary alkyl halide substrates react via the SN1 mechanism while primary substrates react via the SN2 mechanism. While other factors, such as the solvent dictate the mechanism for secondary alkyl halide substrates.
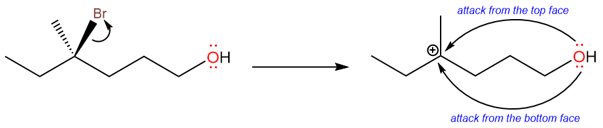
Take an example of (R)-4-bromo-4-methylhexan-1-ol, which gives a racemic ether after the substitution reaction has taken place.
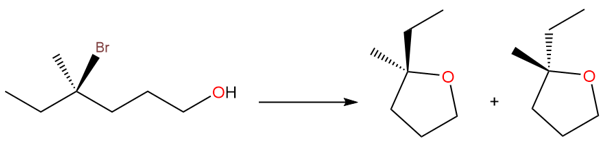
The racemization is due to the formation of a planar carbocation. There is an equal chance that the oxygen atom of the hydroxide can attack from either side of the carbocation, forming a racemic product. The process of racemization is shown below: