Substitution Vs Elimination Summary
The Competition Between Substitution and Elimination Reactions
Substitution and elimination reactions are competing reactions and often a mixture of substitution and elimination products is obtained. This is due to several factors but a major factor is the nature of the nucleophile or the base. Eliminations compete with substitutions because nucleophiles can also act as bases. There are factors, such as the nature of the substrate or the solvent, that direct the outcome of a reaction. But one of the first things to look out for when predicting whether a reaction will be substitution or elimination is the nature of the nucleophile. More specifically, the question of nucleophilicity vs basicity.
Nucleophilicity Vs. Basicity
The basicity of a nucleophile depends upon the charge density or the nucleophile. If a nucleophile is resonance stabilized, its charge density is spread over three or more atoms. These nucleophiles are always weakly basic. An example is the acetate ion:
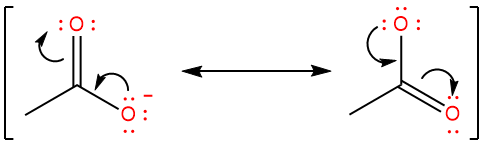
Due to resonance stabilization, the acetate ion is more nucleophilic than it is basic.
In case, where the nucleophile/base is not resonance stabilized, the nucleophilicity/basicity depends upon two things: 1) Electronegativity of the atom carrying the charge/lone pair, and 2) Size of the atom carrying the charge/lone pair. Less electronegative atoms tend to be more basic because of their high charge density. For example, CH3− ion is ten trillion times more basic than the NH2− ion because of the low electronegativity of the carbon atom. As a rule, basicity increases as we move to the left in a period and decreases as we move down in a group.
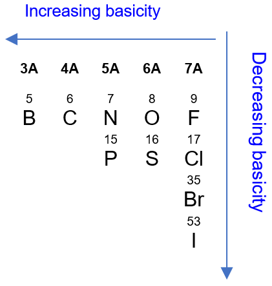
As for the nucleophilicity, it increases down the group with the atomic radius. As atoms become larger, they become more polarizable, more nucleophilic, and less basic.

SN2 Vs E2 Reaction: Different Substrates, Different Reaction Outcomes
Both SN2 and E2 reactions take place with strong nucleophiles and bases. Let’s take an example of the methoxide anion, which is a strong base and a strong nucleophile.
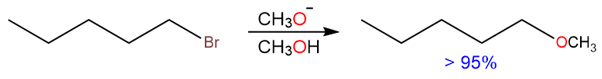
When 1-bromopentane (a 1° alkyl halide) reacts with sodium methoxide, the reaction is almost entirely an SN2 reaction. This is because the substrate is an unhindered primary alkyl halide where the nucleophile has an unhindered approach to the backside attack of the α-carbon.
The same nucleophile, when it reacts with tert-butyl bromide, produces an alkene. The reaction is 100% E2 and no substitution product is formed.
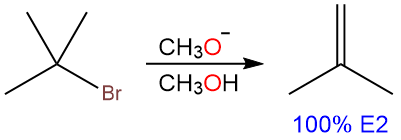
This is because the substrate is a tertiary substrate with a hindered approach to the backside of the α-carbon. So the nucleophile acts as a base and removes a β-hydrogen instead, producing an alkene product.
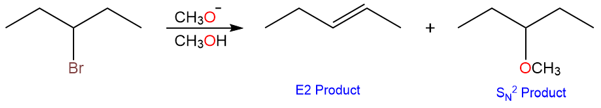
Secondary substrates produce more complex reactions. Both substitution and elimination products are obtained. The proportion of each depends upon other conditions such as the solvent and temperature.
Four Points to Remember
Here are four important points that should help you decide if a reaction would be substitution or elimination.
- Nucleophiles that are only weakly basic.
- If the nucleophile/base is a neutral molecule, it will not react with a primary substrate.
- In case of the substrate is secondary or tertiary, the reaction will be SN1 and E1.
- A high temperature will favor the E1 reaction while a lower temperature will favor the SN1 product.
- Nucleophiles that are strongly basic but not bulky.
- Nucleophiles that are strong bases such as the hydroxide and methoxide will react predominantly via the SN1 mechanism with primary substrates.
- Tertiary substrates will give more substituted (Zaitsev) elimination products via the E2 mechanism.
- Secondary substrates will give a mixture of elimination and substitution products via E2 and SN2 mechanisms.
- Nucleophiles that are strongly basic and bulky.
- Strongly basic and bulky nucleophiles such as the tert-butoxide will preferably give elimination products – even for primary substrates.
- The reaction mechanism will be E2.
- Bulky bases promote the formation of less substituted alkenes (Hofmann products).
- Nucleophiles that are weakly basic and strongly nucleophilic.
- Weakly basic nucleophiles which are either highly polarizable (e.g. I−) or resonance stabilized (e.g. acetate or azide (N3−) always react via the SN2 mechanism with primary substrates.
- Secondary substrates also react with such nucleophiles to give substitution products but the yields are generally low. The reaction mechanism is SN2 in polar aprotic and SN1 in polar protic solvents.
- Tertiary substrates always react with such nucleophiles in polar protic solvents to given SN1 products. Reactions do not usually take place in polar aprotic solvents.
