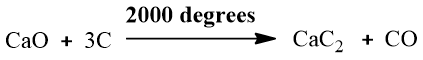Synthesis of Alkynes
Synthesis of alkynes:
Alkynes can be synthesized by the following methods;
1. Elimination:
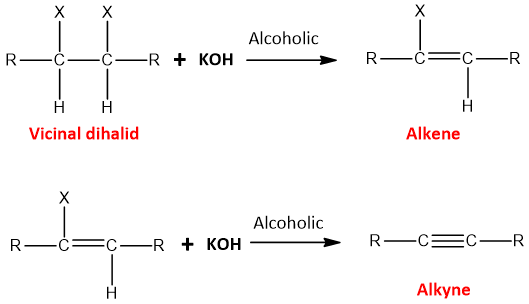
- Dehydrohalogenation of vicinal dihalides:
The compound in which two halogen atoms are attached to two adjacent carbon atoms is called vicinal dihalide.
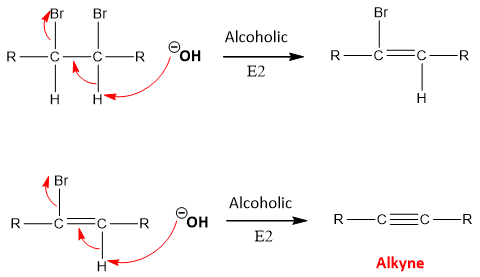
When vicinal dihalides are treated with a strong base like KOH or NaOH, two HX molecules are removed from the compound and two pi-bonds are created. The reaction is carried out in the presence of alcohol as a solvent. The removal of the second HX molecule requires a higher temperature.The reaction is an elimination reaction and is carried out through E2 elimination.
Therefore, the reaction would be concerted and takes place in a single step.
Mechanism:
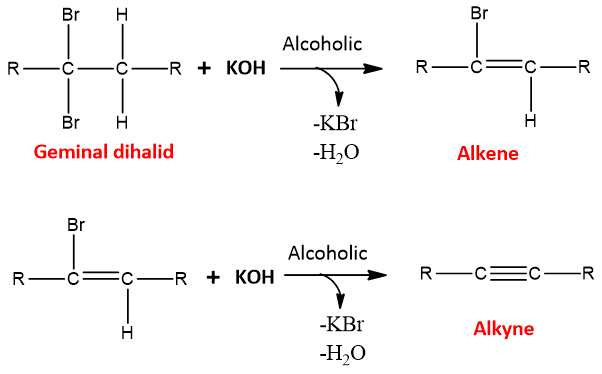
- Dehydrohalogenation of Geminal dihalides:
Geminal dihalides undergo the same E2 mechanism in the presence of a strong base such as sodium amide in liquid ammonia.
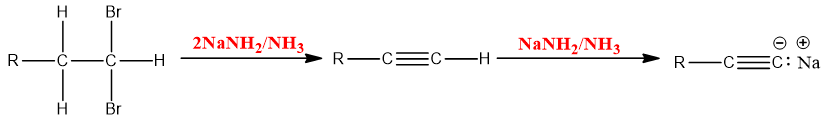
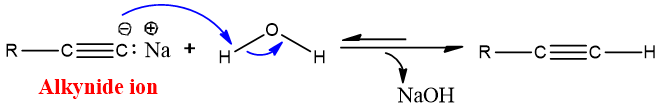
- Synthesis of terminal Alkyne:
Sodium amide is a strong base and is also used for the synthesis of alkynes. Excessive equivalent of sodium amide is used to ensure the formation of elimination product.
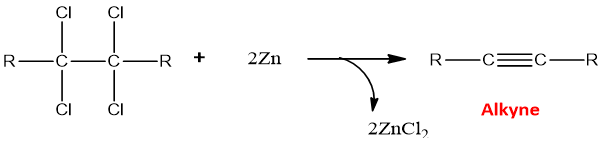
- Dehalogenation of tetrahalides:
The reaction of tetrahalides with metallic zinc results in dehalogenation.
The reaction takes place two times in 2 equivalents of Zn and resulted in the formation of alkyne.
2. Electrolysis:
From unsaturated dicarboxylic acid:
When an aqueous solution of sodium or potassium salts of dicarboxylic acids undergoes electrolysis alkynes are produced. This method is also called Kolbe’s electrolysis.
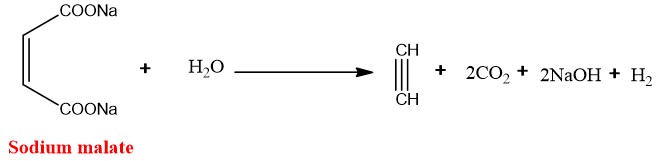
Mechanism:
The salt ionizes in water to sodium or potassium ions and dicarboxylate ion.
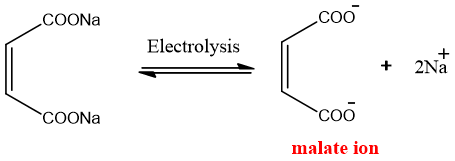
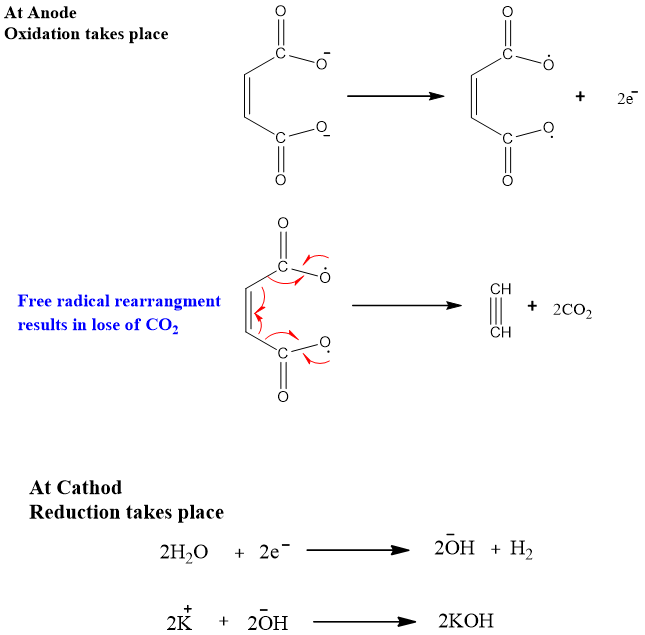
At the cathode, reduction takes place KOH is formed.
3. Industrial method:
Industrially, ethyne (the first member of the alkyne family) is synthesized by the reaction of calcium carbide (CaC2) with water.
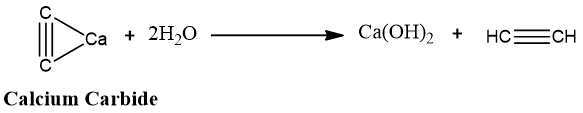
Calcium carbide is synthesized by heating coke and lime in an electric furnace at a high temperature.