Hydration of Alkynes
Hydration Of Alkyne:
The Addition of water to an alkyne is called Hydration. It results in the formation of carbonyl compounds (Aldehyde and ketones).
Alkynes can add water in two ways
- Mercury(II) ion catalyzed direct addition of water(Markonikov product).
- Hydroboration-oxidation based indirect addition of water.
Mercury (II) ion catalyzed hydration:
Alkynes readily undergo hydration in presence of H2SO4 and HgSO4 as catalyst.
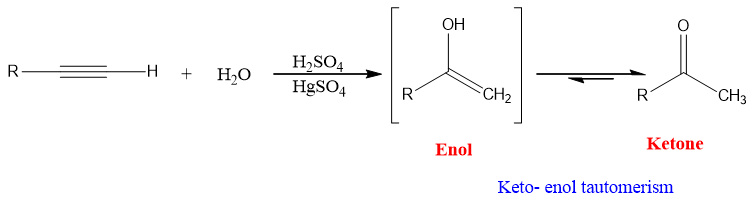
Mechanism:
Alkynes as Nu attacks at electrophilic Hg (II) ion and gives vinylic carbocation. Water then attacks at carbon-bearing positive charge and protonated mercury containing enol is formed which leads to organomercury compound and ultimately neutral enol and ketone.
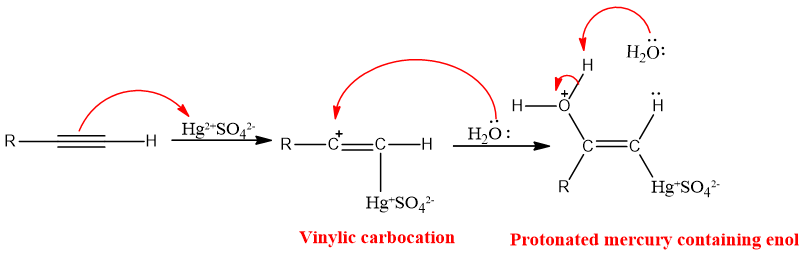
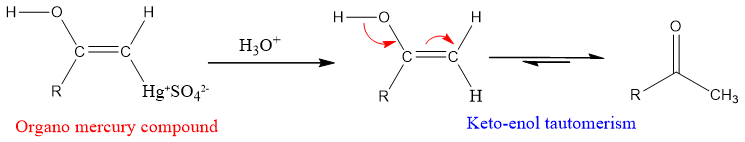
Regio-chemistry:
The addition of water in presence of mercuric sulfate to alkynes follows markonikov’s rule. The negative part of water OH- goes to carbon having less hydrogen and the positive part goes to carbon having more hydrogen, this is linked to the stability of vinylic carbocation.
Drawback:
When an unsymmetrical alkyne reacts with water mixture of products forms so this reaction is more likely for terminal alkynes.
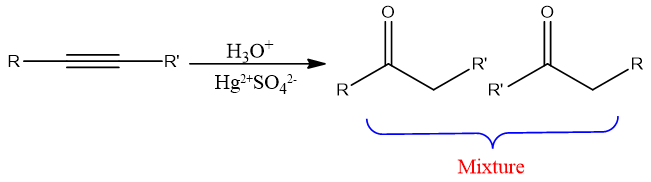
Hydroboration-oxidation hydration:
Alkynes can add boranes like alkenes. Alkynes on reaction with Boranes give vinyl boranes which on oxidation with H2O2 yield enols which then undergo tautomerization and form either aldehyde or ketones depending on the structure of alkyne. Internal alkynes give ketones on hydroboration-oxidation, and terminal alkynes give aldehydes on hydroboration-oxidation.
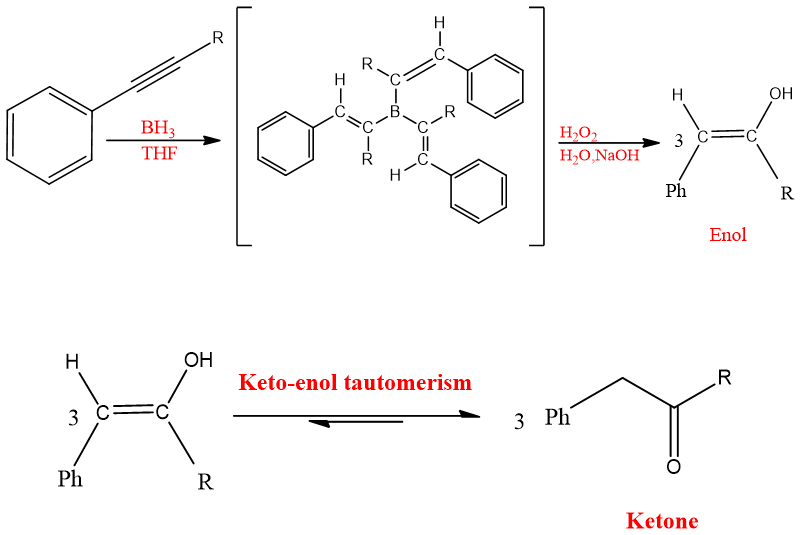
Regio-chemistry:
The addition of water to alkynes involving boranes follows Anti-Markonikov’s rule. In the end product, it seems like the negative part of water OH- goes to carbon having more hydrogen the and positive part goes to carbon having less hydrogen.
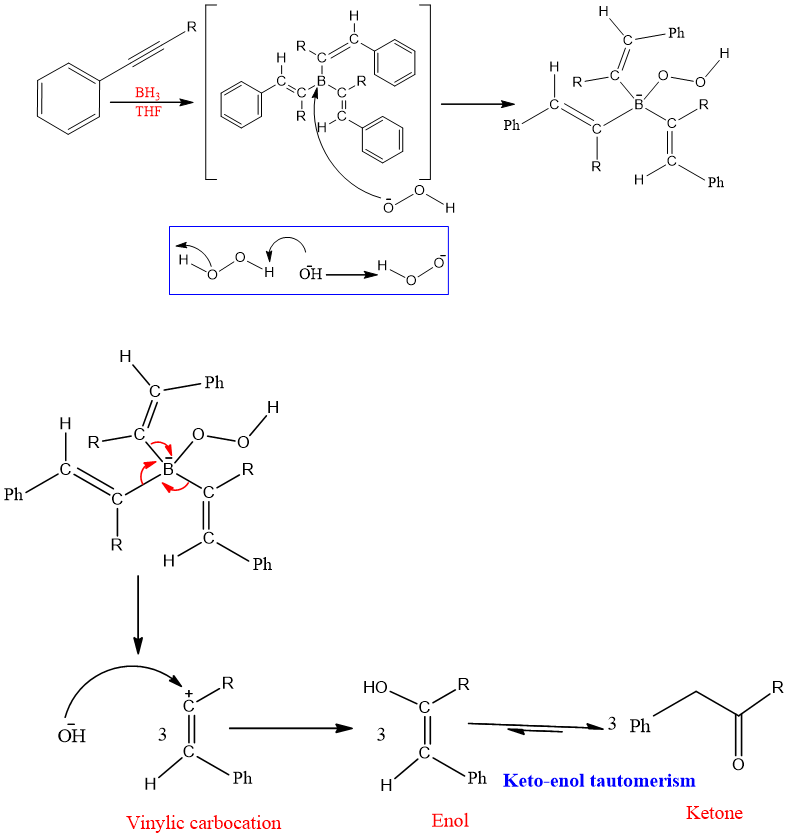
Mechanism:
One molecule of boranes can add three molecules of alkyne and give a triply hydroborated intermediate. This triply hydroborated intermediate then undergoes oxidation with H2O2 and gives vinylic carbocation which on reaction with OH- leads to enol and ultimately ketone.
Comparison between two types of hydration:
Mercury catalyzed hydration is complementary to hydroboration-oxidation hydration due to the difference in their products. Oxymercuration leads to ketones while hydroboration-oxidation leads to aldehydes for a specific alkyne.
