Reduction Reactions with Alkynes (Hydrogenation)
Reduction of Alkynes:
The reduction reaction of alkynes involved hydrogenation.
The addition of hydrogen to alkynes to its pi-bonds is called Hydrogenation.
Alkynes in presence of metal catalyst are reduced to alkanes. Alkynes can be reduced in two ways.
- Complete reduction
- Partial reduction
Complete reduction of alkynes:
Alkynes when treated with H2 in presence of finely divided Ni give alkene intermediate which on further addition of H2 gives alkane. Alkynes can also be completely reduced by using Pd or Pt/C as a catalyst. The catalyst is suspended in the solution that contain alkyne and then exposed with hydrogen gas atmosphere. The reaction occur on the surface of metal and is exothermic. The first addition of H2 is more exothermic than the second addition.
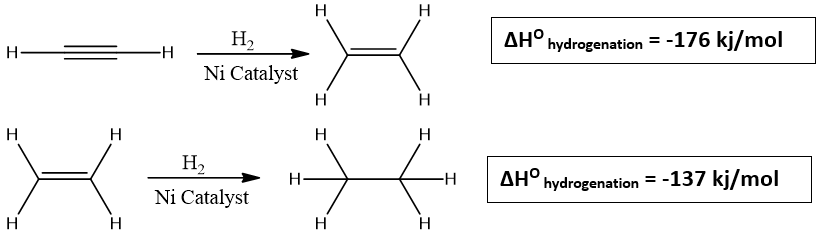
The reaction of hydrogenation is always exothermic because the product formed has less energy than alkyne.
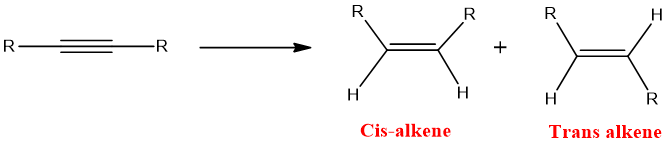
Partial Reduction:
Hydrogenation of alkynes occur in steps and after one hydrogenation i.e., formation of alkenes reaction can be stopped. This type of hydrogenation is called as Partial hydrogenation.
Partial hydrogenation can be carried out in two ways resulting in formation of cis or trans alkene. This can be done by changing catalyst.
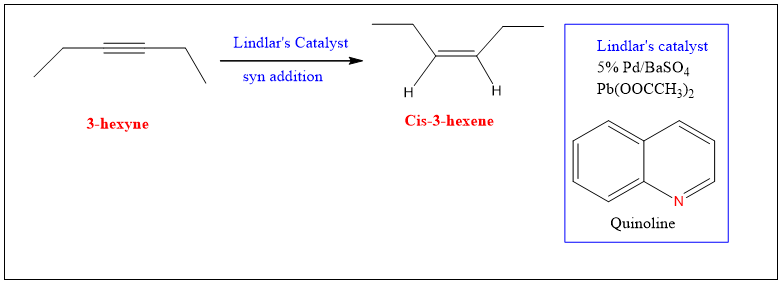
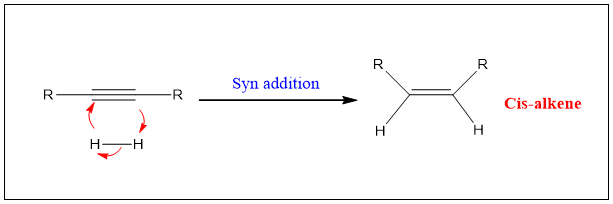
Formation of Cis-alkene:
Alkynes can be converted into cis alkene by using Pd catalyst deposited on barium sulphate or calcium carbonate, treated with lead acetate and poisoned with quinoline. This catalyst is called as Lindlar’s catalyst.
The reaction occur such that the surface of the Pd metal rearranges the Carbon atom such that only one pi bond is reduced. This can happen because of lowering in the efficiency of catalyst because of poisoning.
However, the reaction is stereoselective and both the hydrogen added on same side. Therefore, syn addition takes place and cis product is formed.
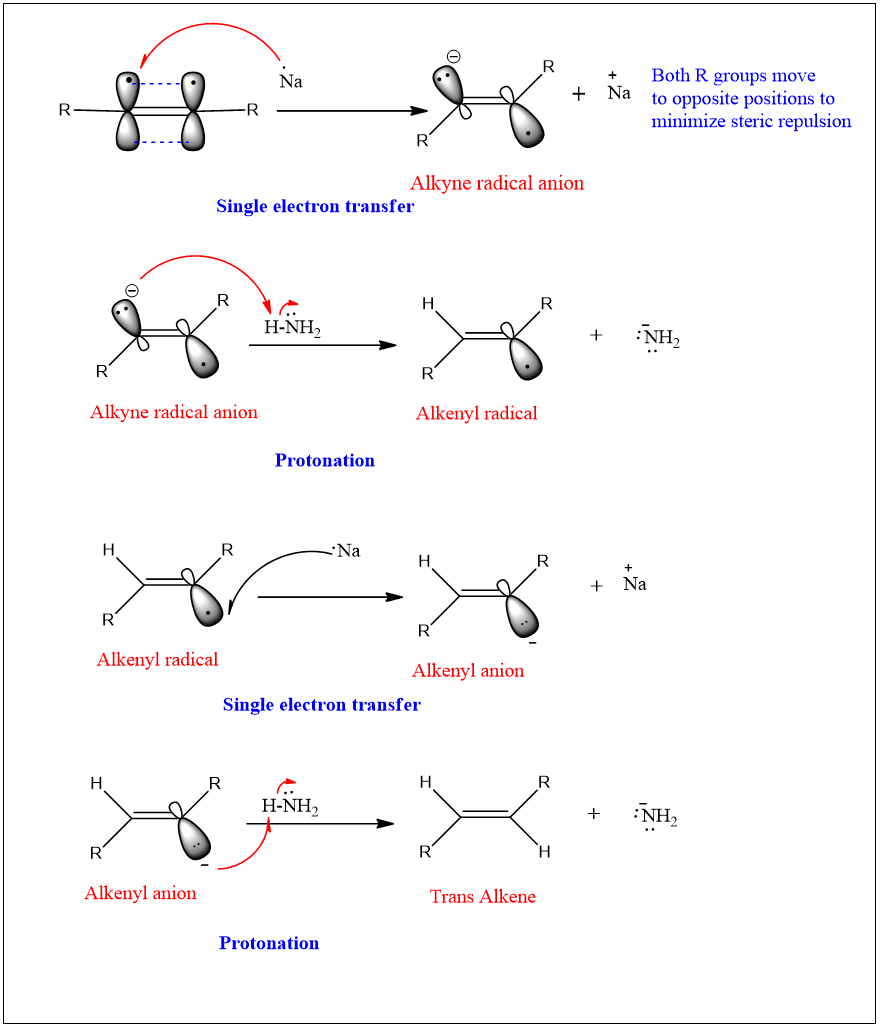
Mechanism:
Synthesis of trans Alkene:
The stepwise one-electron reduction of alkyne results in the formation of trans alkene. This reaction is carried out in the presence of sodium metal dissolved in liquid ammonia. This reduction is also called as dissolving metal reduction. Here the sodium metal in liquid ammonia act as a power full reducing agent and donate electron to alkyne.
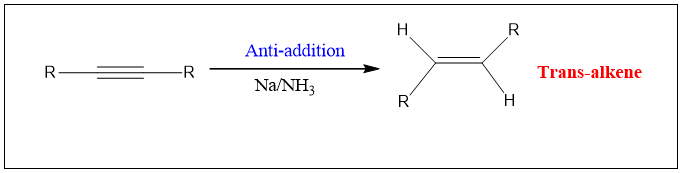
Mechanism:
Step1: Sodium metal in ammonia presents as a sodium ion as it is dissolved. The electron is lost and then transferred to alkyne and an anion is formed.
Step 2: This ion then undergoes protonation by ammonia and an alkenyl radical is formed.
Step 3: This radical then accept another electron from the sodium ion to form an anion again.
Step 4: This anion again undergoes protonation by ammonia and results in the formation of alkene.