Halogenation of Alkynes
Halogenation of Alkynes:
The addition of halogen in the alkynes is called Halogenation.
Halogenation of alkynes is done in the presence of inert solvents like CCl4.Among halogens, F2 reacts fastly and I2 reacts slowly.
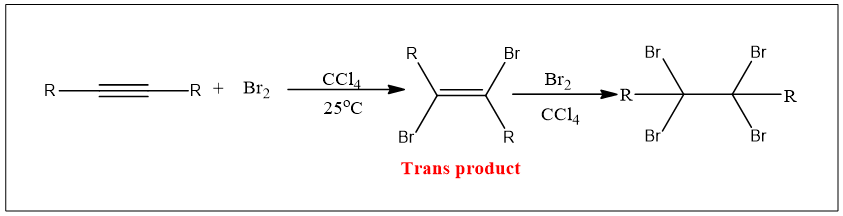
Cl2 or Br2 can be added to alkynes in two steps forming a dihalide and then a tetrahalide. By lowering the temperature reaction can be stopped after the addition of the first molecule of halogen. A trans product is formed.
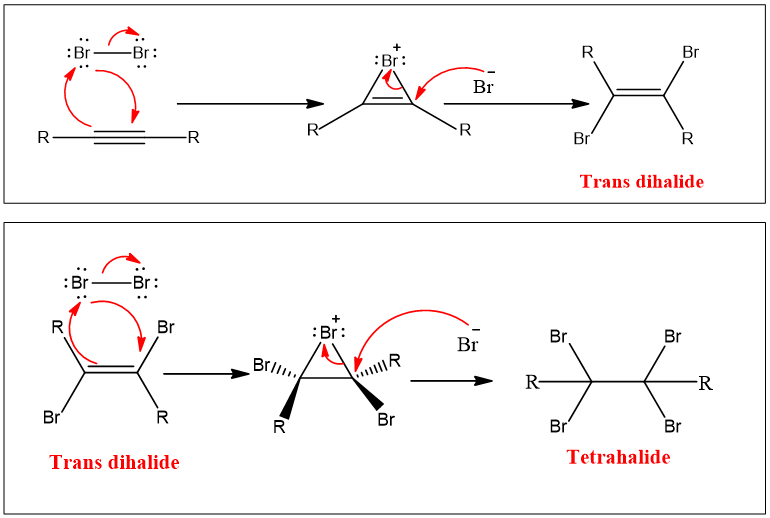
Mechanism:
Br2 molecule becomes polarized when gets closer to π electrons. So π electrons as nucleophiles attack the electrophilic part of the bromine molecule one bromine atom leaves as a bromide ion. An intermediate vinylic bromonium ion is formed. In the next step Br- attack on bromonium ion from back and results in a dihalide. The same steps repeat and a tetrahalide compound is formed. Therefore, the addition of halogen takes place via anti addition, and two successive additions results in tetrahalide.