Oxymercuration of Alkynes
Oxymercuration of alkynes:
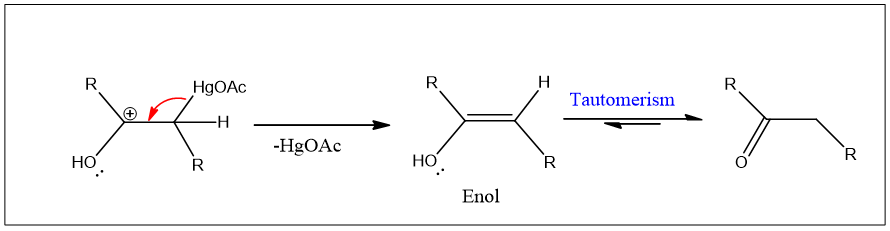
Hydration of alkenes can take place by another reaction which is called oxymercuration of alkynes. Alkynes react similarly to alkenes but the reaction ended up with a product having a double bond i.e., enol product is formed.
This enol product undergoes tautomerism and ketone is formed.
Tautomerism: the isomerism in which isomers differ in the position of the atom and have athesame molecular formula. The isomers are called tautomers of each other. They are readily interconverted to each other. The best example is keto-enol tautomerism in which both isomers have the same M.F. but differ in the position of one hydrogen atom.
Alkyne reacts with mercuric acetate in the acidic environment and enol is formed.
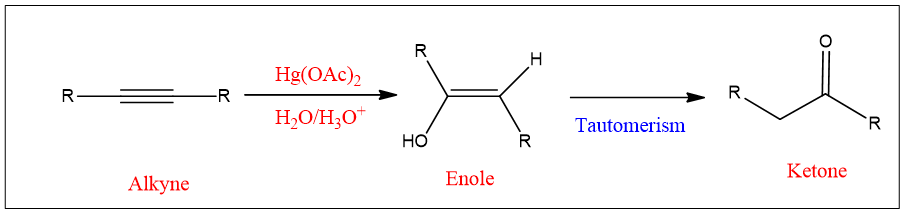
Mechanism:
The mechanism of the reaction is given as;
In the first step mercuric acetate is added to the alkyne through oxidative addition. A 3-membered ring containing a metal cation is formed. Then water molecule attaches to one of the carbons of the resulting ring from the opposite side. These first two steps correspond to anti addition reaction.
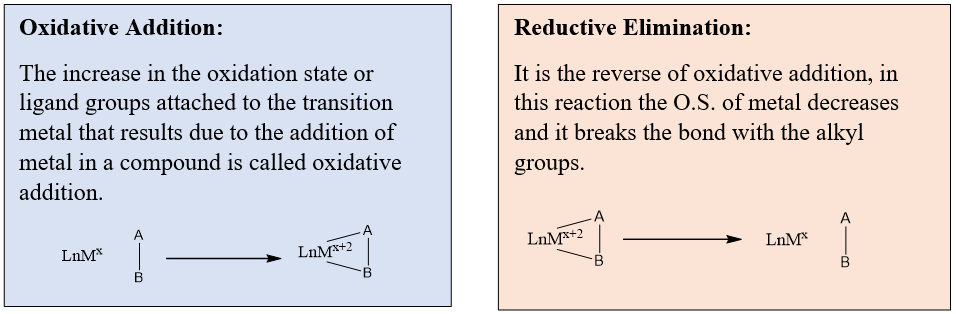
In the next step, a hydrogen atom is added to the other carbon. These steps include indirect hydration of alkyne. On one carbon-hydrogen and on other carbon OH groups will be added. Overall, it becomes the addition of water molecules. This step is completed by the reductive elimination of mercury acetate.
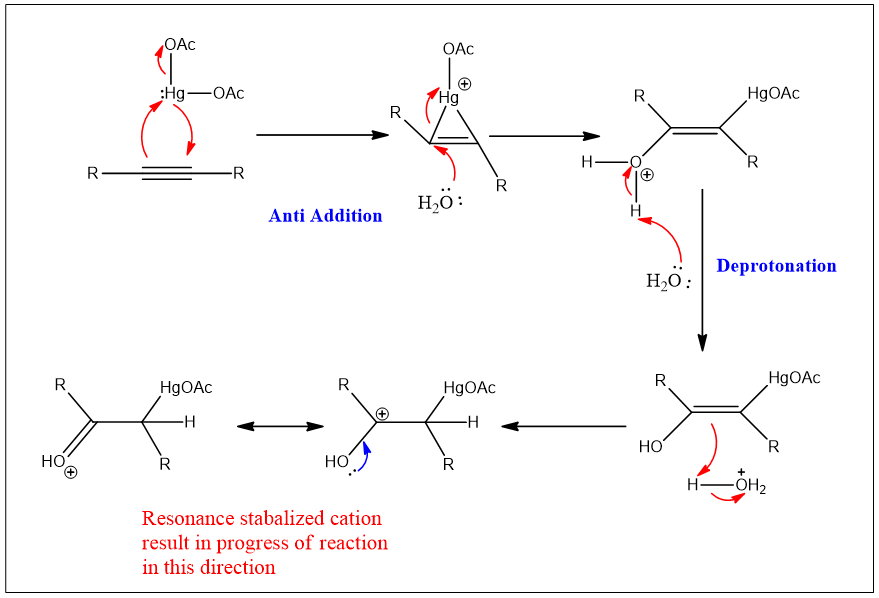
The hydrogen atom adds to the double bond, which results in the formation of a carbocation. That carbocation formed which is resonance stabilized.
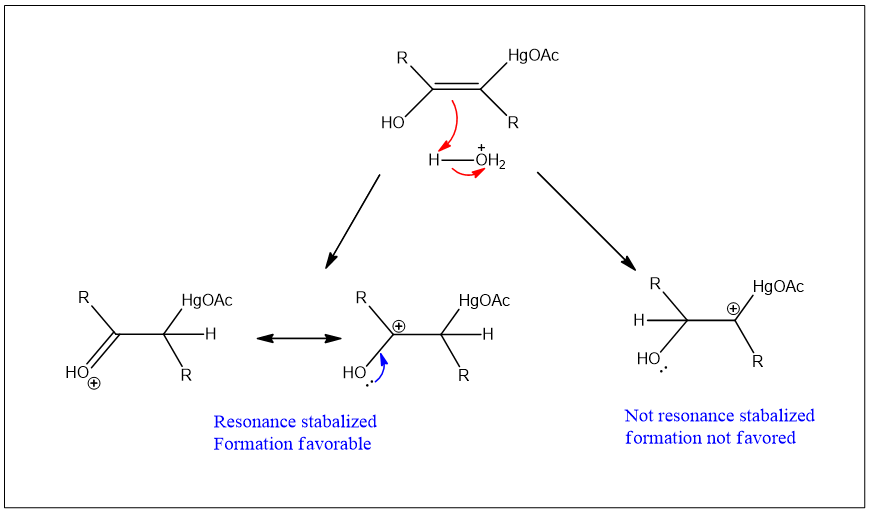
The stabilized carbocation is converted into enol and this enol form is converted into ketone in the last step.