Acetylide Formation
Acetylide:
When the hydrogen of a terminal alkyne or acetylene removed by a base and resulted in the formation of anion connected with a metal like sodium, silver, calcium and copper etc.
The acetylide ion is the carbanionic conjugate base of terminal alkyne.
Acetylides is formed by the deprotonation of terminal alkyne by a strong base. For example, when acetylene reacted with sodium amide (a strong Base), the hydrogen from the sp carbon is removed and an acetylenic anion is formed.
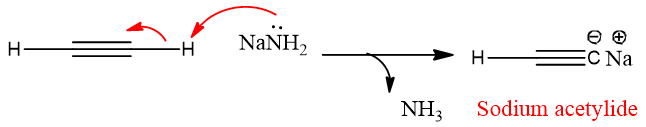
Alkynides formation is only shown by alkynes, it is not the property of alkanes and alkenes. The reason for this is the acidic nature of alkynes. The hydrogen attaches to the sp carbon is acidic in nature because sp carbon has 50% s character in it which makes the carbon more electronegative in nature than sp2 and sp3 carbons. The sp-hybridized carbons pull the shared electrons strongly towards them and make the attached hydrogen loosely bound. This hydrogen becomes acidic and can be removed easily.
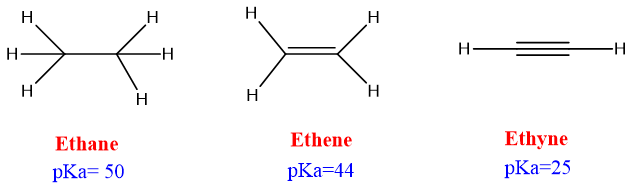
The formation of acetylides or alkynides is shown by only acetylene or terminal alkynes respectively because internal alkynes don’t have acidic hydrogen attached to the sp carbon.
The formation of various metal acetylides can take place as follows;
Formation of alkynides:
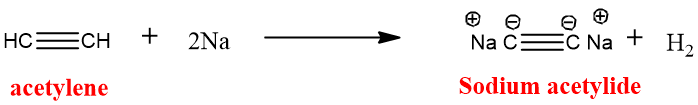
Sodium alkynides obtained when acetylene reacts with sodamide (sodium amide) in liquid ammonia and ammonia gas is released.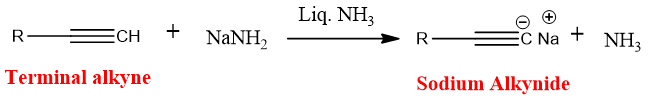
Sodium alkynides can also be formed by passing terminal alkyne of acetylene with molten sodium metal.
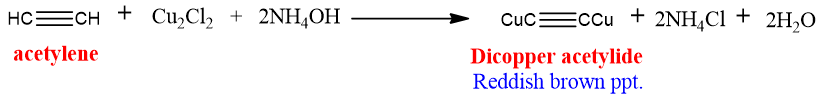
Copper acetylide:
Copper acetylides can be prepared by treating acetylene with the ammonical solution of cuprous chloride. The reddish brown precipitates of copper acetylide are formed.
Silver acetylide:
Acetylene passed through an ammonical solution of silver nitrate and produces white precipitate of disilver acetylide.

Formation of Li alkynides:
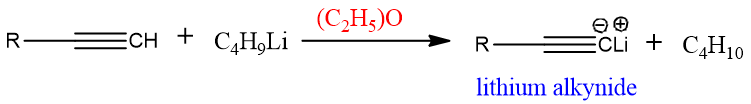
Conversion of alkynides back to alkynes:
on reacting different metal alkynides with dilute mineral acids, alkynes are produced again.

