Substitution Nucleophilic Bimolecular Reactions
Substitution Nucleophilic Bimolecular Reactions:
- The substitution reaction in which a nucleophile replaces an atom or group of atoms in a molecule is called a nucleophilic substitution reaction.
Substrate + Nucleophile à Product + Leaving group
- The reactant on which nucleophile attacks is called the substrate. The substrate in these reactions is an alkyl halide mostly.
- In a substitution reaction the atom or group of atoms that leave when nucleophiles attack the substrate is called as Leaving group.
- Nucleophilic bimolecular substitution reaction is also called an SN2 reaction.
Mechanism:
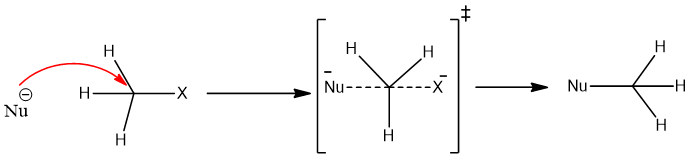
It is a concerted one-step reaction. In this reaction nucleophile attack on the substrate and departure of leaving group takes place in the same step. In this reaction, the extent of bond breaking and bond making is equal and takes place at the same time. The carbon of the alkyl halide is sp3 hybridized and during the reaction is assumed that a transition state exists during the reaction.
The hybridization of carbon in the transition state changes from sp3 to sp2 and therefore becomes a planer. The transition state is hypothetical and it is highly unstable. In the transition state, the formation and breaking of the bond is shown by half bonds (dash bonds).
Features of SN2 Reaction:
Kinetics:
The rate law expression for SN2 reaction is given as;
Rate [Nucleophile] [substrate]
Rate = k[Nucleophile] [substrate]
The rate of reaction is directly proportional to the concentration of substrate and nucleophile. Therefore, a change in concentration of both will affect the rate of reaction. The order of reaction for SN2 reaction is 2 because the rate depends upon the concentration of two reactants.
Product Configuration:
The product of the SN2 reaction has a 100 percent inverted configuration because nucleophiles attack from the opposite side of leaving group.
Energy Diagram for SN2 Reaction:
The energy diagram for SN2 reaction shows that all the reactants i.e., the alkyl group of alkyl halide, nucleophile, and leaving group are present in the transition state. The dashed bonds in the transition state show partial bonds formed between nucleophiles and carbon, and a partial bond is broken between carbon and leaving group. The charges on Nu and X in the bracket show the substantial partial charges.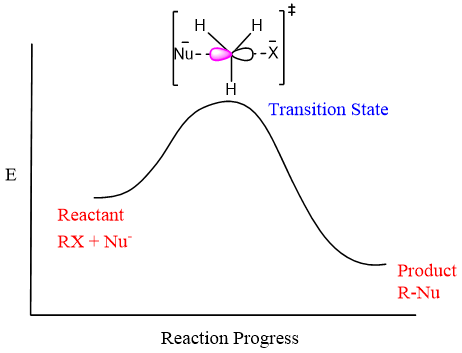
Nature of Substrate:
The best substrate for SN2 reaction is primary alkyl halide.
Nature of Solvent:
SN2 reactions can take place in non-polar, polar aprotic solvents and the gas phase.
Factors Affecting rate of SN2 reaction:
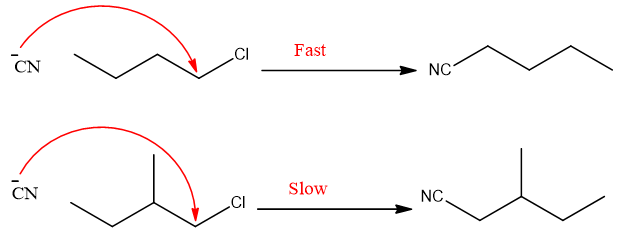
- 1. Nature of substrate:
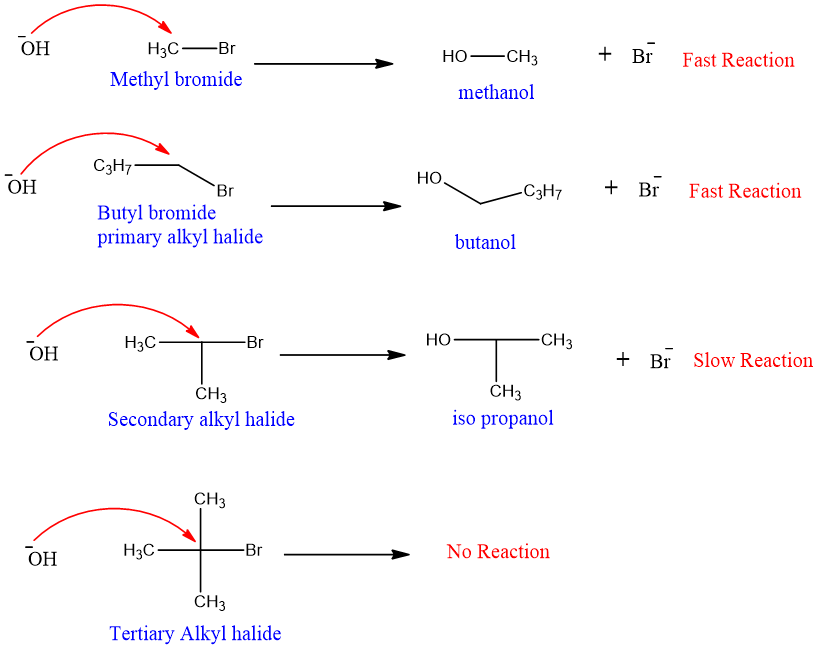
Substituents on neighboring carbon in primary alkyl halide also affect the rate of an SN2 reaction.
- 2. Nature of Nucleophile:
The nature of nucleophiles also affects the rate of reaction. Generally, the stronger the nucleophile faster the reaction.
For example, from thiol and alcohols, thiols are stronger nucleophiles therefore reactions with thiols are faster than with alcohol.
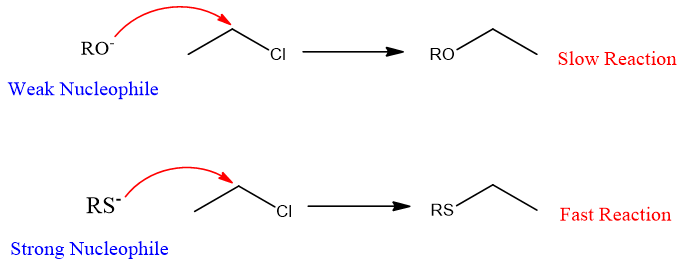
- 3. Effect of Solvent:
Solvent nature greatly affects the rate of an SN2 reaction. In polar solvents, the reaction slows down because polar solvents like water solvate the nucleophile. Therefore, nucleophilic power decreases, and the rate of reaction decreases.
The best solvent in which SN2 reactions are fast is a polar aprotic solvent. These solvents lack hydrogen. Example of polar aprotic solvents is acetone, acetonitrile, nitromethane, and dimethyl sulfoxide.
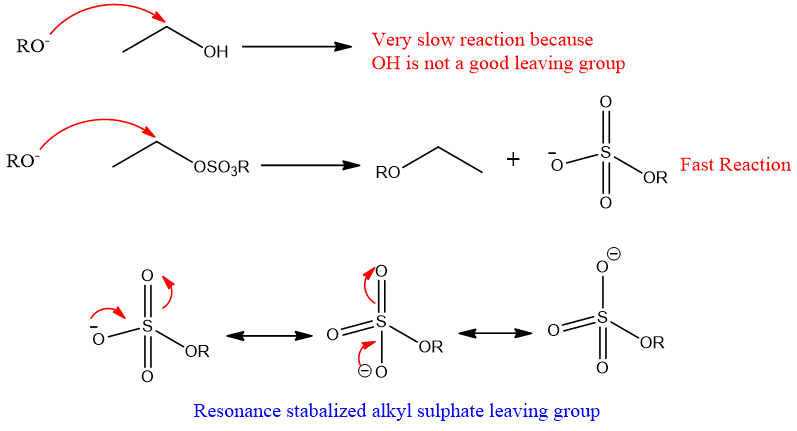
- 4. Effect of Leaving the group:
Leaving a group greatly affects the rate of an SN2 reaction. If the leaving group is weak then it will not remove easily and therefore reaction rate becomes slow.
Generally, the larger the size of leaving group greater its ability to remove.
For example. Among halogens, iodine has a larger size when it leaves iodide ions will form, and due to the larger size, the negative charge will delocalize and become stable. On the other hand, fluorine forms fluoride ions which will have a smaller size, and therefore the negative charge has a very small electron cloud for delocalization as compared to iodine. So, iodine is a good leaving group as compared to fluorine.
The power of good leaving a group of halogens is give
I->Br- > Cl- > F-
Similarly, the leaving groups that stabilized by resonance after leaving are good leaving group as compared to those that cannot stabilized by resonance.