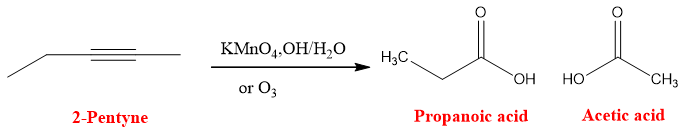
Oxidative Cleavage of Alkynes
Oxidative Cleavage of alkynes:
Oxidative cleavage of alkynes is the breakdown of both pi-bonds and sigma bonds i.e., the cleavage of the triple bond. The oxidative cleavage of alkyne results in the formation of carboxylic acid. The oxidative cleavage can be used to locate the position of the triple bond present in the compound.
Oxidative cleavage of alkynes can be done by:
- Ozonolysis
- Oxidation with KMnO4
Ozonolysis:
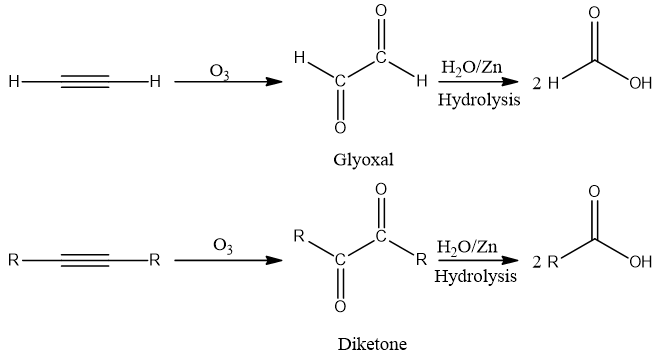
The cleavage of unsaturated bonds in alkenes, alkynes, and azo compounds by ozone (O3) is called ozonolysis. Oxidation of alkynes by ozone yields acid anhydride or diketones. Acid anhydride on hydrolysis gives carboxylic acids. Fragmentation in alkynes is incomplete as compared to alkenes. On aqueous work-up acid anhydride or diketone undergoes hydrolyzation which results in carboxylic acids. Ozonolysis is used to locate the position of the triple bond.
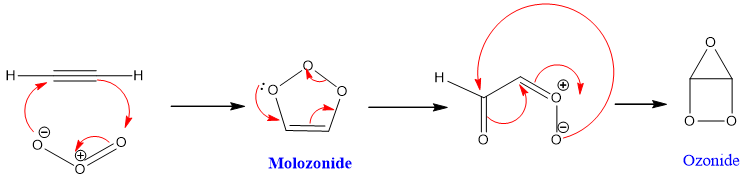
Mechanism:
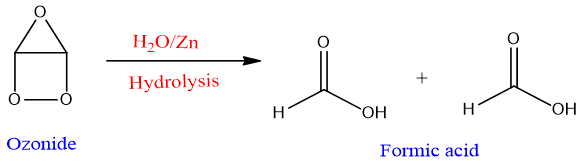
Alkynes on reaction with ozone get cleave and give ozonide intermediate. Ozonide on aqueous work up in presence of Zn metal gives di-carbonyl compound.
Oxidative cleavage using KMnO4:
Oxidative cleavage of alkynes results in the formation of carboxylic acids. The carbon of the triple bond converted into carbonyl system and an OH group is attached to the carbon. Basic potassium permanganate solution is used and then work up by adding water because the product of the reaction is an acid and it will convert into carboxylate salt in basic medium. To convert the salt back to acid water is added. Oxidative cleavage of terminal and internal alkyne results in different products.
Oxidative cleavage of internal alkyne:
The products of internal alkyne oxidation are carboxylic acids.
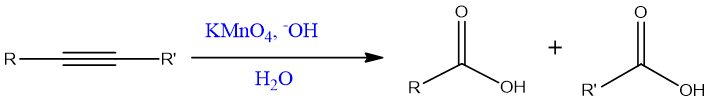
Mechanism:
KMnO4 gives MnO4- ion which adds to alkyne. The reaction goes by syn addition.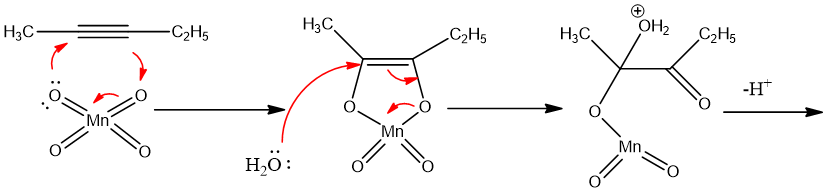
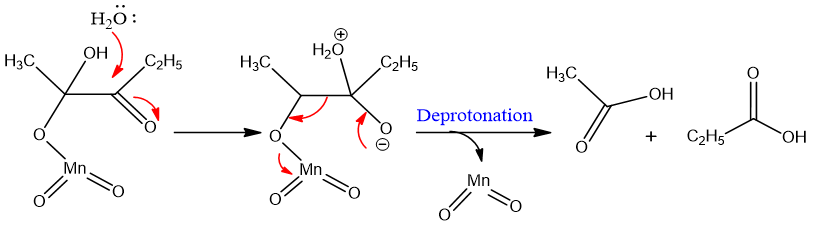
The product of terminal alkynes are carboxylic acid and carbon dioxide.
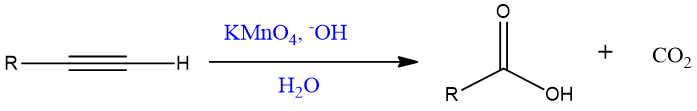
Mechanism:
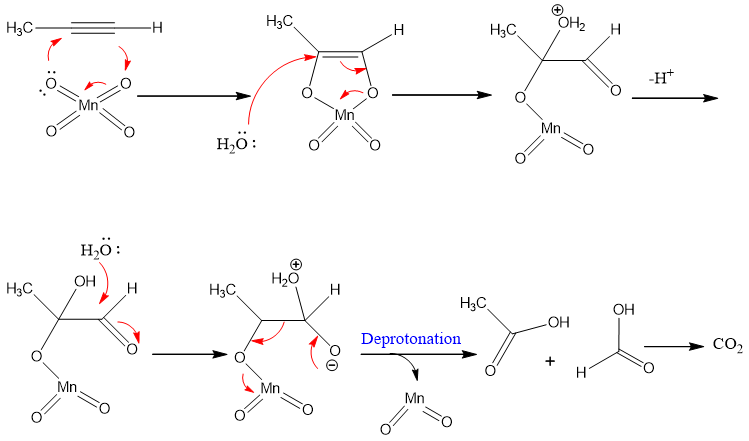
The pink color of KMnO4 discharge due to formation of brown ppt. of MnO2.
Determination of Position of triple bond in the compound:
Oxidative cleavage can be used for the determination of triple bond in the compound. By knowing the nature of the product, it can be determined either the alkyne was terminal or internal. If both the products are carboxylic acid than the alkyne was internal and if Carbon dioxide is one of the products, then alkyne was internal.
Similarly in internal alkyne position of triple bond can be determined by knowing the exact structure of products. For example, if product of oxidation of alkyne are propanoic acid and acetic acid then it suggests that the triple bond will be at carbon two position. It can be determined easily by imagining the bond between two carboxylic acids by joining the carbonyl carbons and removing OH attached to the carbons.
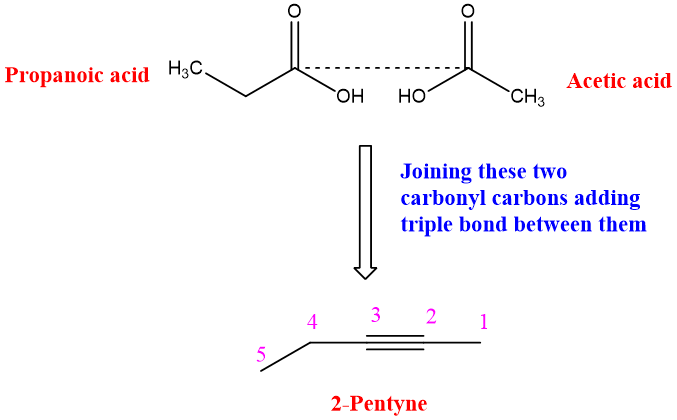
The reaction takes place as: