Addition of HX of Alkynes
Addition of HX of Alkynes
Alkyne Reactions:
Alkynes are susceptible to electrophilic addition reactions like alkenes. The difference is, that alkenes add 1 equivalent of reagent while alkynes add 2 equivalent of reagent. Alkynes need drastic conditions as compared to alkenes. Electrophilic addition reactions in alkynes occur through complex mechanistic pathways. It matches alkenes to some extent like the first attack on the positive portion of adding reagent as a nucleophile. The difference is alkynes involve vinylic carbocation and alkenes involve alkyl carbocation. A vinylic carbocation is highly unstable that’s why alkynes need drastic conditions to proceed with the reaction.
Reaction with HX:
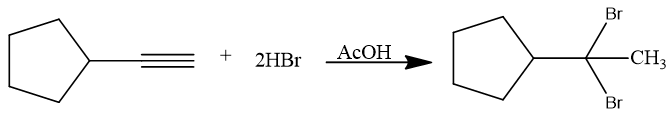
Alkynes on reaction with HX in presence of AcOH (acetic acid) lead to dihalide product. The reaction can be stopped when 1 equivalent of reagent was added but on the excess of reagent (2 equivalent), a dihalide product is formed.
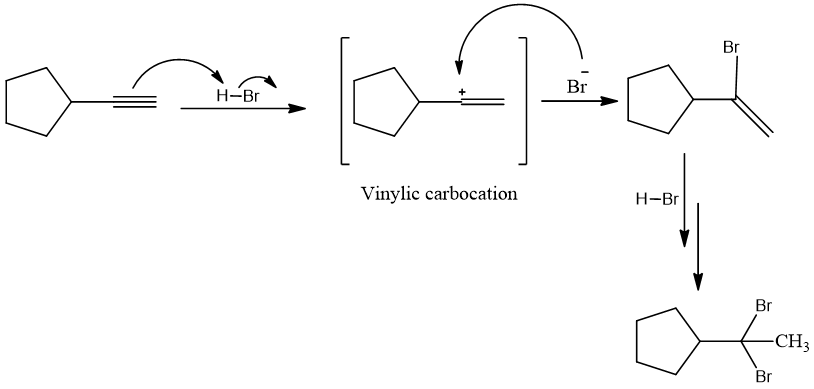
Mechanism:
π electrons of triple bond as nucleophile attack on positive part of HBr and vinylic carbocation is formed as intermediate. Negative part of adding reagent then attack back on carbon bearing positive charge. Same steps are repeated for second molecule of HBr to be added. The dihalide product is always a geminal dihalide.
Regio-chemistry:
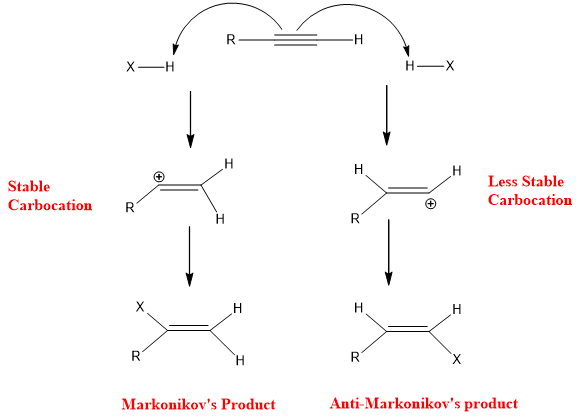
The addition of HX to alkynes follows the Markonikov rule, the negative part of adding reagent goes to that carbon having less hydrogen, and the positive part of adding reagent goes to that carbon having a greater number of hydrogens. Markonikov product is related to the stability of vinylic carbocation.
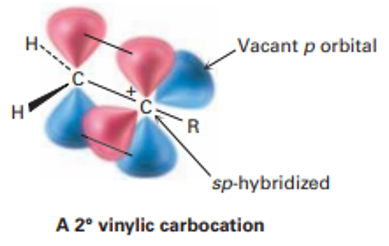
Stability of vinylic carbocation:
The only possible vinylic carbocation to be formed in alkynes is 2o vinylic carbocation. 1o vinylic carbocation has an equal chance to be formed like 2o but it readily converts to relatively stable 2o vinylic carbocation.
A vinylic carbocation is highly unstable as it is present on sp hybridized carbon atom which is already electron deficient due to greater s character, further electron depletion makes it highly unstable.
2o vinylic carbocation vs 1o vinylic carbocation:
Empty ‘p’ orbital on vinylic carbocation causes unwanted destabilization. If any electron donating group is attached it can diminish its destabilizing effect by unsaturated C-based/heteroatomic substituent or by hyperconjugation of attached CH3, CH2, and CH.
In the case of 1o no such substituent can be attached so secondary vinylic carbocation is more likely to be formed than primary.