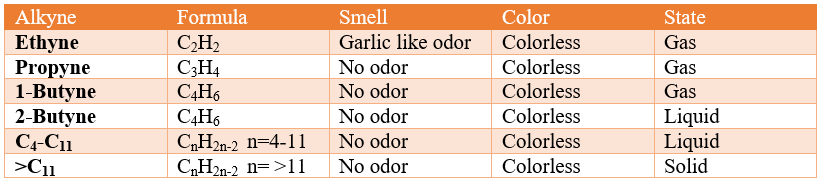
Properties of Alkynes
Alkynes:
The class of organic compounds that contain at least one triple bond is called Alkynes.
They have the general formula CnH2n-2. A triple bond is also called an acetylenic bond because the first member of the series is acetylene or ethyne.
Properties of Alkynes:
Physical properties:
- All members of alkynes are odorless and colorless except acetylene which has a characteristic garlic-like smell. The pure acetylene is odorless, commercial grade acetylene has this garlic like odor which is due to presence of calcium carbide impurities.
- The first three members of the series are gaseous in nature, members from C4-C11 are liquid and all others above C11 are solid. Butyne has two isomers; 1-butyne and 2-butyne. From these two isomers, 1-butyne is gas and 2-butyne is liquid at room temperature.
- Boiling point and melting point increases with size and molecular weight. Similarly, melting and boiling point of alkynes are higher than the corresponding alkenes and alkanes.
- The members of this series are soluble in organic solvents as they are non-polar so dissolve in non-polar solvents like benzene, toluene etc.
- Densities increases gradually as molecular size increases.
Chemical Properties:
- Alkynes contain triple bond having one sigma bond and two pi bonds.
- The C-H bond in alkyne is slightly polar, it is because the carbon atom in alkyne exhibit sp hybridization. It has 50 % s-character. Due to this, the size of orbitals around sp hybridized carbon become smaller and electron density become closer to the carbon atoms. Thus, carbon becomes slightly electronegative and the hydrogen atom is loosely bound to this carbon. Therefore, C-H bond becomes slightly polar than alkenes or alkanes.
- Moreover, there is high electron density present between the two carbon atoms, these two carbon atoms come closer to each other. The electron density is not exposed in case of alkynes which makes them less reactive as compared to alkenes.
- They are less reactive towards electrophilic reactions as compared to alkenes.
- Alkynes also show acidic properties, as the H atom bound to carbon of triple bond is slightly polar so it can be removed easily by using a base and they form alkylides.
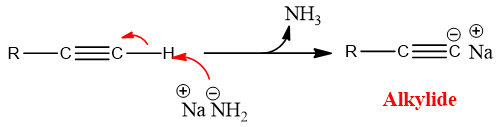
This acidic property is only possessed by terminal alkynes or acetylene as they have acidic hydrogen i.e. the hydrogen which is attached to sp carbon.
Due to this acidic property they can be act as nucleophile.
- The C-C bond length in alkynes becomes less as compared to alkene and alkanes. C-C bond length in alkynes is 120pm.
