Nomenclature of Amides
Nomenclature of Amides
Amides are the derivatives of carboxylic acids. The -OH group of carboxylic acid is replaced by -NH2 group.

Amides are classified as primary, secondary and tertiary amides. In primary amides the nitrogen atom is bonded to one carbon (carbonyl carbon). In secondary amides or N-substituted amide the nitrogen atom is bonded to two carbon atoms. In tertiary amides or N,N-substituted amides the nitrogen atom is bonded to three carbon atoms.
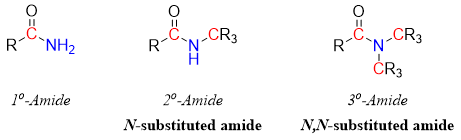
Naming primary amides:
Primary amides are named by replacing the -ic acid or -oic acid of the carboxylic name with -amide. For example
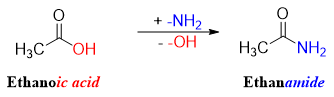
Amides derived from carboxylic acids named alkanecarboxylic acids are named by using a suffix carboxamide. Following are some examples of naming primary amides systematically.

Naming secondary amides:
Secondary amides are named as primary amides. The single alkyl group on nitrogen atom is taken as substituent and its position is specified by the prefix N-.
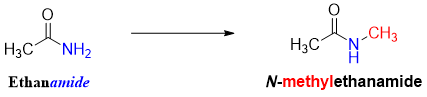
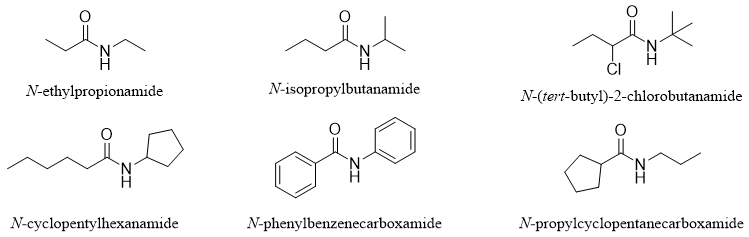
Following are some examples of naming secondary amides systematically.
Naming tertiary amides:
Tertiary amides are named as secondary amides. The two substituents on nitrogen atom are specified by the prefix N-. If both substituents are identical then the prefixes N,N- and di- are used. If the substituents are different, then each substituent is specified with separate N- in alphabetical order.

Following are some examples of naming tertiary amides systematically.

Naming cyclic amides (Lactams):
Lactams are cyclic amides derived from amino acids. The amino group and carboxylic group of amino acids join to form lactams.

Systematically lactams are named in two ways. First, lactams are named as 2-azacycloalkanones where aza specifies nitrogen atom at position 2 to carbonyl group. Secondly, lactams are named by adding the term lactam at the end of the IUPAC name of corresponding amino acids.

