Amide Reduction (making Amines)
Amide Reduction (making Amines)
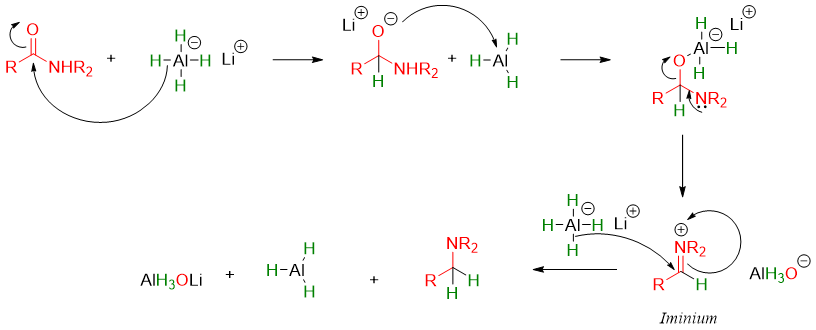
Like esters, amines also undergo two successful addition of hydride ions to produce corresponding amines. Overall, the carbonyl group (C=O) of amide is converted to methylene group (-CH2-) when reacted with lithium aluminum hydride (LiAlH4). Like esters and carboxylic acids, amides cannot be reduced by NaBH4. Aluminum atom being more electropositive than boron shifts electron density towards hydride ion making it more reactive.
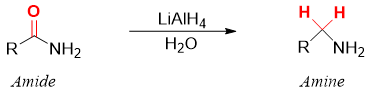
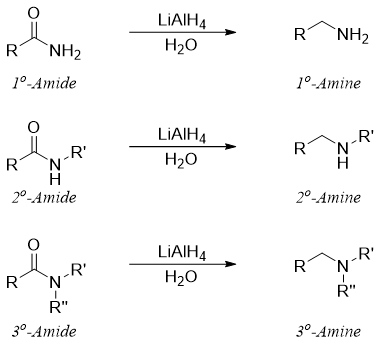
The reduction of primary amides produces primary amines, secondary amides produce secondary amines and tertiary amides produce tertiary amines.
The mechanism of this reaction involves the formation of imines. The imine further reacts with LiAlH4 to produce corresponding amine.
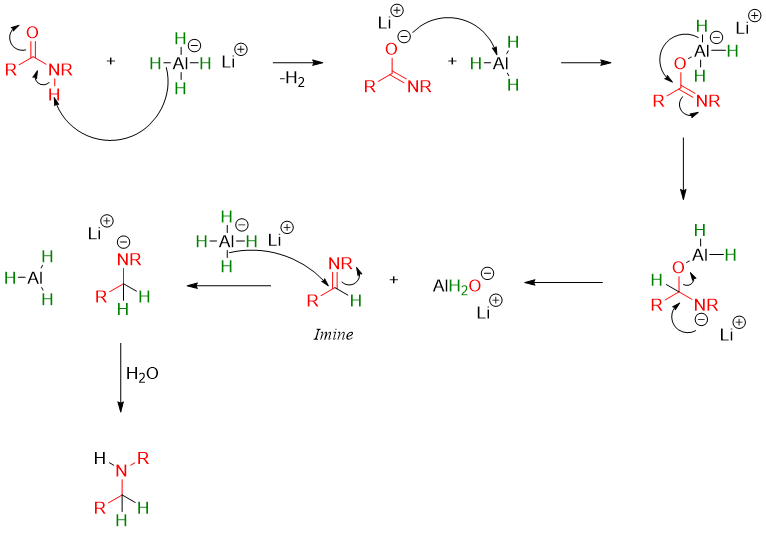
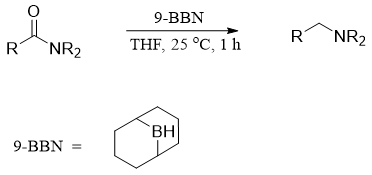
The mechanism of reduction of N,N-disubstituted amide is different but the final product is the same.
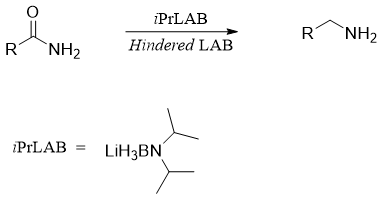
There are many other reagents used in organic synthesis to carry out reduction of amides to corresponding amines. These reagents include Lithium aminoborohydride (LAB) and 9-Borabicyclo[3.3.1]nonane (9-BBN). The reactivity of LAB is comparable to LiAlH4. However, LAB have some advantages over LiAlH4. LAB are air stable, thermally stable, non-pyrophoric and only hydrolyze in protic solvent at pH above 4.
9-BBN is also used to reduce amide into amines. It is a mild reducing agent, and it does not form complex with tertiary amines, therefore, a stoichiometric amount of 9-BBN is sufficient to reduce amide into amines.