Amide Formation from Acid Anhydrate
Amide Formation from Acid Anhydride
Acid anhydrides are the second most reactive carboxylic acid derivatives. The leaving group of acid anhydrides is the carboxylate ion. Acid anhydrides react with an amine to produce an amide and the carboxylate ion.
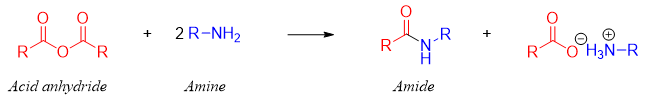
Acid anhydrides when reacted with ammonia produces primary amides, with primary amines produces secondary amides and with secondary amines produces tertiary amides.
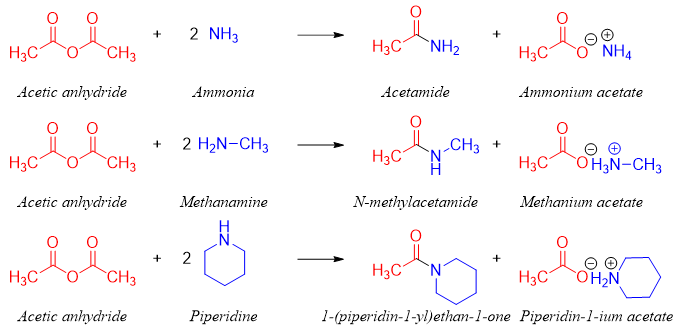
In this reaction acid anhydride is reacted with two moles of amine to make sure the presence of sufficient amine to react with both carbonyl groups of the acid anhydride. Furthermore, the second equivalent of amine also reacts with the proton produced during the reaction as amines are stronger bases. This reaction follows addition-elimination mechanism.
First Mechanism: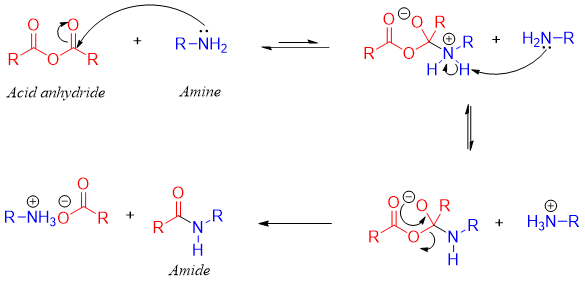
Second Mechanism:
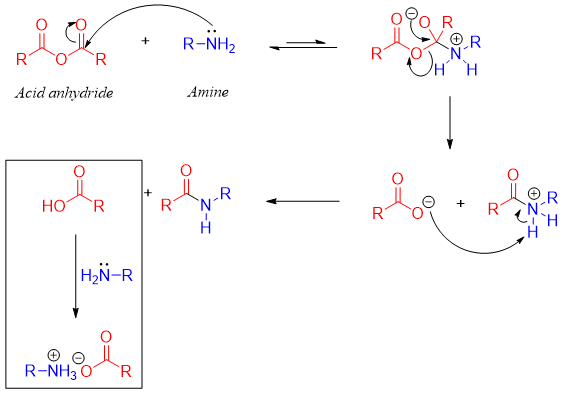
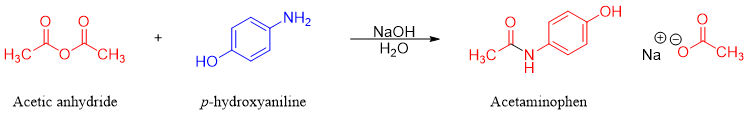
Acid anhydrides are generally used to prepare N-substituted amides (secondary amides). For example, an analgesic drug called acetaminophen is synthesized by reacting 1 mole of acetic anhydride with 1 mole of p-hydroxyaniline in the presence of NaOH.